Year: 2003 Vol. 69 Ed. 5 - (10º)
Artigo Original
Pages: 649 to 655
Myringoplasty using a new biomaterial allograft
Author(s):
José Antonio Apparecido de Oliveira1,
Miguel Angelo Hyppolito2,
Joaquim Coutinho Netto3,
Fátima Mrué4
Keywords: myringoplasty, tympanic, membrane, biossintetic allografts, growth factors
Abstract:
Myringosplaty is a surgery that is performed to control infections in middle ear, to reconstruct the sound conducting mechanism for the oval window and protection of the round window. Some materials are used to reconstruct the tympanic membrane like autologous temporalis fascia, cartilage-perichondium graft taken from the tragus, cartilage only, dura mater human placental graft. It is important the vascular support to the graft with well-vascularized flap in wide perforations. Aim: The main goal of this work is shown the use of a new biomaterial, the natural latex membrane with polylysin. Study design: Longitudinal Cohort. Material and method: This material was developed from Department of Biochemistry from Faculdade de Medicina de Ribeirão Preto Universidade de São Paulo. The natural latex membrane is used stimulating neovessels and organized tecidual growing in different parts and tissues of the human body. This material promotes any allergic reaction and is innocuous to the human tissue. The 238 ears with tympanic membrane perforation resulted from chronic middle ear infection and thek was going through myringoplasty with autologous temporalis fascia and natural latex membrane. The ages were 7 to 76 years. Results: The first results show 181 (90,5%) healing tympanic membrane perforation, 96 healing of wide perforation 73 medium perforation and 12 small one. Neovessels could be seen in all grafts and remaining tympanic membrane. This is not a habitual funding when the natural latex membrane is not used. Conclusion: According to these results we can conclude that the natural latex membrane can be used as a temporary implant in myringoplasty, improving the well vascularized of remaining tympanic membrane.
![]()
Introduction
Normal tympanic membrane is a simple organ that presents two basic functions: transmission of sound pressure to the oval window and sound protection of the round window, in addition to protecting the middle ear against infections 1. The differences in dimensions between the surface of the tympanic membrane and stapes footplate increases, by hydraulic principles, the auditory level by 27 dB 1, 2.
Histologically, the tympanic membrane has 3 layers: an epithelial layer, comprising two rows of epithelial cells; an intermediate thin layer of fibrous constitution, with collagen fibers, fibroblasts and endothelial cells, and a medium layer of mucous nature, which is a continuum with the middle ear mucosa 3-7.
The tympanic membrane can have its anatomical structure impaired, leading to perforation, which can be permanent or temporary. It can be damaged by middle ear pathologies, associated with perforation in acute and chronic otitis media, granulation tissue and traumas, including myringotomy with perforation after removal of ventilation tubes 8. Many studies report as an important factor for permanently maintaining the anatomical affection of the tympanic membrane the impairment of the intermediate layer, affecting the organization of the collagen fiber architecture. Others refer to the epithelial abnormalities as the most important factor, stating that the epithelial reconstruction is the support to the repair of the intermediate collagen layer 5-7, 9-11.
Different methods are described since its is a tympanic perforation, including the classical technique of myringoplasty and tympanoplasty, using grafts made of temporal muscle fascia, perichondrium of the tragus, endothelial tissues, dura, and compatible biomaterials, such as human or bovine placenta collagen film, all of them using classical techniques of microsurgery to conduct the plastic procedure of the tympanic membrane 2, 12-18.
There is a current tendency in simplifying the modern microsurgical procedures used to reconstruct the tympanic membrane and improve their efficacy 2, 19-22.
It has been described the use of epithelial growth factors and fibroblasts, successfully studied in the healing process of different tissues, and more recently, in tympanic membrane perforation, involving simple techniques. The acquisition of these growth factors is possible by recombinant DNA techniques that are very complex 21-32.
The positive results of experimental studies in animals using growth factors in closing tympanic membrane perforations have been encouraged in clinical studies with patients with chronic otitis media. A study conducted by Hamsay (1995) using the epidermal growth factor in tympanic membrane perforation in humans did not show satisfactory efficacy concerning their closure 23, 34, 26-35.
Some studies demonstrated the action of keratinocyte lysate cultures in healing of different tissues, and more recently, its action in healing and closure of tympani perforations in animal models. Clinical trials conducted in patients submitted to classical techniques of myringoplasty using grafts of temporal muscle fascia and culture of keratinocytes showed considerable reduction of the closure time of the perforations, but the way it acts is still uncertain and it suggests the effect is related to primary mitotic activity through growth factors and cytokines 27, 36-38. The use of hyaluronic acid has also been tried, which distributes to the extracellular matrix in the connective tissue of men and other animals, presenting positive results in tympanic perforation, especially small and traumatic perforations, in the absence of chronic middle ear infection proposed as the substitute use of classical techniques of microsurgery to reconstruct the tympanic membrane in these cases 20-22, 39, 40.
A new biocompatible material based on natural polymerized latex (natural latex extracted from Hevea brasiliensis) with polylysin 0.1% was developed by a method of cold polymeralization in the division of neurochemistry of the Department of Biochemistry, Medical School, Ribeirão Preto, University of Sao Paulo. It was initially used as an inductor material of healing of damaged esophageal walls. We observed in the neoformed esophagus of dogs (area of 4-6cm) a marked increase in vascularization (angiogenesis), epithelialization (pseudo-stratified epithelium), submucous glandular neoformation and neoformed muscle fibers 25, 41, 42. The material does not cause allergic reaction and it is not rejected by the body of dogs or humans. In other areas of medicine, it has been successfully studied and used in the healing process of phlebopathic, arterial and diabetic ulcers and in burned body surfaces.
Motivated to search for a simple and practical method whose results would improve myringoplasty, we tried to use the natural latex membrane with polylysin 0.1% as adjuvant in closing tympanic perforations caused by middle ear infection, without cholesteatoma, trying to find a material that could serve as the support for the natural process of regeneration of the remaining tympanic membrane. The classical techniques of myringoplasty using temporal muscle fascia grafts proved to be unsuccessful, which ranged from 10% to 30%, depending on the size of the perforation and the technique used, with residual perforation especially resultant from lack of vascularization of the free graft 43-47. Based on the fact that a latex biomembrane favors neovascularization, we decided to study the effect of efficacy of the vascularization of the temporal fascia graft based on the remaining membrane, following the conventional techniques of myringoplasty and looking for more positive results with the surgery.
Objectives
The objectives of the study were to observe the interaction of the latex biomembrane with the human tympanic tissue, to observe the biocompatibility of the latex biomembrane, toxicity of the biomaterial, presence of allergic reactions, possibility of the use of natural membrane of latex as temporary implant in myringoplasty, effect of this stimulation in the success of myringoplasty, and to observe the neovascularization induced by the biomaterial.
Material and Methods
We randomly selected 238 patients registered and followed up in the outpatient unit of Otorhinolaryngology who had chronic perforation of the tympanic membrane and clinical history of simple chronic otitis media, with intermittent chronic otorrhea for over one year. None of them presented history of traumatic rupture of the tympanic membrane.
The size of perforations was divided into wide (> than one quadrant of the tympanic membrane), medium (from halt to one quadrant of tympanic membrane) and small (smaller than half quadrant of the tympanic membrane), subjectively assessed. The selected ears did not present signs of infection, nor otorrhea (200). Perforations were filmed and photographed preoperatively with an otoendoscope (Richards 235210-2.7mm-0º).
We excluded 38 ears of the 238 initial ears considering the following exclusion criteria:
- postoperative infection - 10
- presence of glue ear - 1
- presence of cholesteatoma - 1
- polyp - 3
- poor positioning of latex - 23
TOTAL = 38
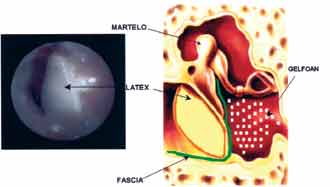
The patients were submitted to classical techniques of myringoplasty, performed by a team of surgeons and resident physicians of the unit, under general anesthesia and using free temporal muscle fascia graft, supported by gel foam placed in the middle ear and under the margins of the scarified perforation. Later, we placed the natural latex membrane externally, supported by the lateral aspect of the margins of the remaining tympanic membrane and in contact with the temporal fascia. The plate was supported by gel foam and otological ointment containing polymixin and bacitracin (Figure 1).
The patients were discharged from hospital one day after the surgery after otomicroscopic assessment for early diagnosis of infection (presence of otorrhea). We did not prescribe otological drops with antibiotics in these patients as routine, but only when the cases showed signs of infection.
On the 7th postoperative day the patients were submitted to a second assessment with otomicroscopic inspection of the operated ear, checking the occurrence of infection (otorrhea), presence of ointment in the external auditory canal and positioning of latex biomembrane. In this period, if the latex biomembrane was loose it was repositioned, if there was presence of otorrhea, it was treated with topical otological drops of ciprofloxacin, and these ears were excluded from the final assessment (exclusion criteria).
The patients were assessed 30 days later, in which we checked the same parameters once again. In this period, the latex biomembrane was removed and the aspect of the graft was observed and documented by otoendoscopy, as well as the success of the tympanic perforation closure.
Results
Table I shows the number of ears and the age range considered in the study.
Out of the total surgeries considered to be successful, with complete temporal fascia incorporation, we describe in Table II the characteristics of this group concerning gender, affected side, location and size of the impaired tympanic membrane and number of unsuccessful tympanoplasties previous to the latex biosynthetic membrane application.
- Positive results with incorporation of graft - 181 ears - 90.5%
- Negative results - 19 ears - 9.5%
- Surgical team - faculty and resident physicians
- Exuberant vascularization and hyperemia of the graft and remaining tympanic membrane
- Mean time for confirmation of graft incorporation - 28 days
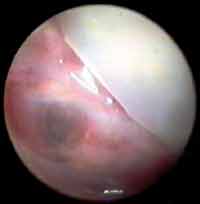
The first aspect observed was the status of the graft, emphasizing the exuberant vascularization and hyperemia of graft and remaining tympanic membrane (Figures 3, 4 and 5). Such fact was not observed in the ears submitted to myringoplasty without placement of the natural latex membrane, in which the graft was pale and little vascularized.
The second observed aspect was the success of perforation closure, in which we detected it in 90.5% (181 cases), emphasizing that 32 ears had already been submitted to one surgery, 6 ears had undergone 2 surgeries and 3 ears, 3 unsuccessful myringoplasties. We also noted complete closure of 96 of the ears in which perforations were wide.
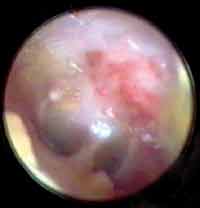
The mean time for closure of perforations was 28 days. Two months later, tympanic membranes had already shown normal aspect (Figures 6 and 7).
Figure 1. Photo showing latex biomembrane with polylysin.
Figure 2. Graphic demonstration of the position of the temporal muscle fascia and the latex membrane close to the tympanic membrane.
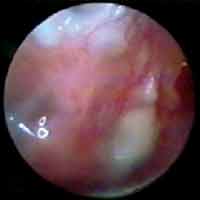
Figure 3. Otoendoscopy of the patients on postoperative day 45, showing intensive hyperemia and vascularization of the temporal muscle fascia graft, incorporating the remaining tympanic membrane.
Figure 4. Otoendoscopy of the patients on postoperative day 25, showing the neoformed vessels from the remaining tympanic membrane.
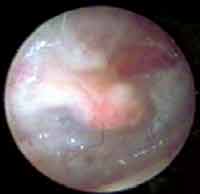
Figure 5. Otoendoscopy of the patient 2 weeks after the surgery, showing vascularization of the graft right after the removal of the latex membrane.
Figure 6. Otoendoscopy of the patient on postoperative day 10 showing closed perforation (only using the membrane) and latex membrane.
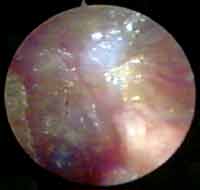
Figure 7. Otoendoscopy of the patient two months after the surgery showing the normal aspect of the tympanic membrane (after closure of the middle perforation).
Discussion
The processes involved in the closure mechanisms of the tympanic perforations are still not fully understood 9-11, 27, 31, 48. Studied conducted in the two past decades pointed to the importance of the growth factors of a normal tympanic membrane and the expression of these factors occur differently in perforated tympanic membranes 23-25.
Such studies strengthened the expectations of clarifying the factors that act in healing and closing damaged tympanic membranes and determine the different healing behavior compared to other tissues 9-11, 27, 31.
Experimental studies in animals proved to be favorable to the use of epithelial growth factors and fibroblasts, stimulating the cell growth and promoting vasodilation, with increase of blood supply to the tympanic membrane, favoring the closure of perforations 49. However, in the long run, in animal studies, it has demonstrated the possibility of developing cholesteatoma and there was no successful closure of chronic perforations in human studies 28, 29, 35, 36, 38.
The tendency to try to simplify the traditional techniques to correct tympanic perforations, reducing costs and operational aspects and making them completely effective, stimulates the search for alternatives to new grafts and to improve those currently available, especially those of temporal muscle fascia which are frequently used and whose success rates range from 70% to 90% 43-47.
The latex natural membrane with polylysin used in this study proved to be an efficient alternative in healing the tissues with possible action of the complex mechanisms of repair of the tympanic membrane. It is a low cost and easy to acquire material, does not cause allergic reactions or rejection by human body. Since it is a vegetal material there is no concern about possible virus transmission such as hepatitis C and B and HIV, which happens in collection from growth factors and lysates of keratinocytes that require human donating cells.
Preliminary studies showed the presence of possible vascular vegetal growth factors in the species Hevea brasiliensis and in action in human tissues, leading to the inference that its capacity induce angiogenesis, improve vascularization of grafts and temporal muscle fascia, based on the remaining tympanic membrane, which happened in our study 50.
Table I. Description of the studied group.
Ears - 238 considered in the study
Ears - 38 excluded by the exclusion criteria
Ears - 200 considered for the final assessment
Ears - 181 considered as successful surgery
Ears - 19 considered as unsuccessful surgery
Age 7 - 76 years (mean of 24 years)
Table II. Characterization of the studied group in which the results were positive with complete incorporation of the graft.
Perforation - Location
Central - 118
Central anterior - 17
Central inferior - 4
Central superior - 3
Posterior superior - 9
Posterior inferior - 10
Anterior inferior - 20
Total - 181
Size of the Perforation
Small - 12
Medium - 73
Wide - 96
Total - 181
Previous unsuccessful surgeries
1st surgery - 32
2nd surgery - 6
3rd surgery - 3
Total - 41
Conclusions
The latex natural membrane produced from Hevea brasiliensis proved to have excellent interaction with human tympanic membrane tissues, excellent biocompatibility, no toxicity and absence of allergic reactions.
The efficiency in regenerating perforated tympanic membranes occurs especially due to the intensive process of revascularization of the remaining membrane.
Another important factor observed in the study was that the success in closing the tympanic perforation with latex biosynthetic membrane as adjuvant was significant also when the surgery was performed by resident physicians in Otorhinolaryngology, which is going to be compared to the surgical results of resident physicians procedures before the use of latex biosynthetic membrane.
References
1. Wullstein H. Theory and Practice of Tympanoplasty. Laryngoscope 1956; 66:1076-95.
2. Briggs RJ, Luxford WM. Chronic Ear Surgery: A Historical Review. Am J Otol 1994; 15:558-67.
3. Amoils CP, Jackler RK, Milczuk H, Kelly KE, Cao K. An Animal Model of Chronic Tympanic Membrane Perforation. Otolaryngol Head Neck Surg 1992; 106:47-55.
4. Johnson A, Hawke M. The Function of Migratory Epidermis in the Healing of Tympanic Membrane Perforations in Guinea Pig. Acta Otolaryngol (Stockh) 1987; 103:81-6.
5. Litton WB. Epidermal Migration in the Ear:The Location and Characteristics of the Generation Center Revealed by Utilizing a Radioactive Desoxyribose Nucleic Acid Precursor. Acta Otolaryngologica (Suppl.) 1969; 240:5-39.
6. Reijnen CJH, Kuijpers W. The Healing Pattern of the Drum Membrane. Acta Otolaryngologica (Suppl.) 1971; 287:1-74.
7. Taylor M, McMinn RMH. Cytology of Repair in Experimental Perforations of the Tympanic Membrane and its Relationship to Chronic Perforations in Man. TR Am Acad Ophth Otol 1967; 71:802-12.
8. Jackson CG, Schall DG, Glasscock ME 3rd, Macias JD, Widick MH, Touma BJ. A surgical Solution for the Difficult Chronic Ear. Am J Otol 1996; 17:7-14.
9. Spandow O, Hellstrom S, Dahlstrom M. Structural Characterization of Persistent Tympanic Membrane Perforations in Man. Laryngoscope 1996; 106:346-52.
10. Gladstone HB, Jackler RK, Varav K. Tympanic Membrane Wound Healing. An Overview. Otolaryngol Clin North Am 1995 Oct; 28:913-32.
11. Koba R. Epidermal Cell Migration and Healing of the Tympanic Membrane:An Immunohistochemical Study of Cell Proliferation Using Bromodeoxyuridine Labeling. Ann Otol Rhinol Laryngol 1995; 104:218-25.
12. Carrat X, Bozon N, Rabaud M, Deminiere C, Bebear JP. Development of Artificial Collagen-elastin Conjunctive Matrix for Tympanoplasty. Concept and 1st Results of Animal Experiments. Ann Otolaryngol Chir Cervicofac 1995; 112:52-7.
13. Ensari S, Selampinar F, Akalin Y, Kara CO, Dagli S, Ergin A, Ozdem C. The Use of Molded Tympanic Heterograft (Lamb Peritoneum). Ear Nose Throat J 1995 Jul; 74:487-9.
14. Grote JJ, Bakker D, Hesseling SC, Van Blitterswijk CA. New Alloplastic Tympanic Membrane Material. AM J Otol 1991; 12:329-35.
15. Hartwein J, Leuwer R. "Crown-cork tympanoplasty": A Method for Complete Reconstruction of the Tympanic ¨Membrane Laryngorhinootologie. 1992; 71:102-5.
16. Klotz G, Flesch J, Bilger P. Use of New Materials in Tympanic Reconstruction. Rev Laryngol Otol Rhinol (Bord) 1988; 109:67-9.
17. Truy E, Disant F, Morgon A. Experimental Study in Dogs of a Human Reticulated Placental IV Collagen Film for Myringoplasty. Rev Laryngol Otol Rhinol (Bord) 1990; 111:79-82.
18. Truy E, Disant F, Tardy M, Tayot JL, Eloy R, Morgon A. Use of Human Placental Type IV Collagen in Tympanoplasty. Results of Initial Animal Experimentation. Ann Otolaryngol Chir Cervicofac 1990; 107:209-13.
19. Kohn FE, Feijen J, Feenstra L. New Perspectives in Myringoplasty. Int J Artif Organs 1984; 7:151-62.
20. Laurent C, Soederberg O, Aniko M, Hartwig S. Repair of Chronic Tympanic Membrane Perforations Using Applications of Hyaluronan or Rice Paper Prosthesis ORL J Otorhinolaryngol (Relat. Spec.) 1991; 53:37-40.
21. Stenfors LE. Repair of Traumatically Ruptured Tympanic Membrane Using Hyaluronan. Acta Otolaryngol 1987 (Stockh) (Suppl) 442:88-91.
22. Stenfors LE. Treatment of Tympanic Membrane Perforations With Hyaluronan in an Open Pilot Study of Unselected Patients. Acta Otolaryngol (Stockh) 1987 Suppl; 442:81-7.
23. Fina M, Bresnick S, Baird A, Ryan A. Improved Healing of Tympanic Membrane Perforations with Basic Fibroblast Growth Factor. Grow Factors 1991; 5:265-72.
24. Mondain M, Saffiedine S, Uziel A. Fibroblast Growth Factor Improves the Healing of Experimental Tympanic Membrane Perforations. Acta Otolaryngol (Stockh) 1991; 111:37-341.
25. Mrue F, Thomazinni JA, Tambeliini H, Coutinho Netto J, Ceneviva R, Lachat JJ, Zborowski AC. Latex Membrane from Hevea brasiliensis as a biomaterial-A New Concept for an Old Material. J Biomaterials - In Press.
26. O'Daniel TG, Ptitjean M, Jones SC et al. Epidermal Growth Factor Binding and Action on Tympanic Membranes. Ann. Otol Rhinol Laryngol 1990; 99:80-4.
27. Somers T, Goovaerts G, Schelfhout L, Peeters S, Govaerts PJ, Offeciers E. Growth Factors in Tympanic Membrane Perforations. Am J Otol 1998; 19:428-34.
28. Dvorak DW, Abbas G, Ali T, Stevenson S, Welling DB. Repair of Chronic Tympanic Membrane Perforations With Long-Term Epidermal Growth Factor. Laryngoscope 1995; 105:1300-4.
29. Hom DB. Growth Factors in Wound Healing. Otolaryngol Clin North Am 1995 Oct; 28:933-53.
30. Vrabec JT, Schwaber MK, Davidson JM, Clymer MA. Evaluation of Basic Fibroblast Growth Factor in Tympanic Membrane Repair. Laryngoscope 1994; 104:1059-64.
31. Koba R, Kawabata I. Immunohistochemical Study of Transforming Growth Factor- Expression in Normal and Perforated Tympanic Membrane. Ann Otol Rhinol Laryngol 1995; 104:793-7.
32. Friedman N, Wrigth CG, Pawlowiski KS, Meyerhoff WL. Effect of Basic Fibrolast Growth Factor on Perforated Chinchilla Tympanic Membranes. ENT-Ear, Nose & Throat Journal 1997; 76:559-64.
33. Mondain M, Ryan A. Epidermal Growth Factor and Fibroblast Growth Factor Are Induced in Guinea-pig Tympanic Membrane Following Traumatic Perforation. Acta Otolaryngol (Stockh) 1995; 115:50-4.
34. Mondain M, Ryan A. Histological Study of the Healing of Traumatic Tympanic Membrane Perforation After Basic Fibroblast Growth Factor Application. Laryngoscope 1993; 103: 312-8.
35. Ramsay HA, Heikkonen EJ, Laurila PK. Effect of Epidermal Growth Factor on Tympanic Membranes With Chronic Perforations: a Clinical Trial. Otolaryngol Head Neck Surg 1995; 113:375-9.
36. Somers T, Duinslaeger L, Delaey B, Verbeken G, Vanhalle S, Boedts D, Govaerts PJ, Offeciers E. Stimulation of Epithelial Healing in Chronic Postoperative Otorrhea Using Lyophilized Cultured Keratinocyte Lysates. Am J Otol 1997; 18:702-6.
37. Clymer MA, Schwaber MK, Davidson JM. The Effects of Keratinocyte Growth Factor on Healing of Tympanic Membrane Perforations. Laryngoscope 1996; 106:280-5.
38. Somers T, Verbeken G, Vanhalle S, Delaey B, Duinslaeger L, Govaerts P, Offeciers E.Lysates From Cultured Allogeneic Keratinocytes Stimulate Wound Healing After Tympanoplasty. Acta Otolaryngol (Stockh) 1996; 116:589-93.
39. Amoils CP, Jackler RK, Lustig LR. Repair of Chronic Tympanic Membrane Perforations Using Epidermal Growth Factor. Otolaryngology Head and Neck Surgery 1992; 107:669-83.
40. Hellstromp S, Laurent C. Hyaluronan and Healing of Tympanic Membrane Perforations. An Experimental Study. Acta Otolaryngol (Stockh) 1987 Suppl; 442:54-61.
41. Mrue F, Coutinho Netto J, Ceneviva R, Zucolotto S, Lachat JJ, Zborowski AC. Esophageal Replacement by a New Kind of Esophageal Prosthesis. XXX World Congress of the International College of Surgeons. Kyoto, Japan November 1996; 247-52.
42. Mrue F. Substituição do Esôfago Cervical por Prótese Biossintética de Látex - Estudo Experimental em Cães. Tese de Mestrado. Faculdade de Medicina de Ribeirão Preto-USP-1996.
43. Chandrasekhar SS, House JW, Devgan U. Pediatric Tympanoplasty. A 10-year Experience. Arch Otolaryngol Head Neck Surg 1995; 121:873-8.
44. Halik JJ, Smyth GD. Long-Term Results of Tympanic Membrane Repair. Otolaryngol Head Neck Surg 1988; 98:162-9.
45. Perrin A, Mendoza R, Baril C, Receveur M, Roulleau P. Long-term anatomic results of tympanic homografts. Apropos of 170 cases. Ann Otolaryngol Chir Cervicofac 1987; 104:535-9.
46. Podoshin L, Fradis M, Malatskey S, Ben-David J. Tympanoplasty in Adults: A Five-year Survey. Ear Nose Throat J 1996; 75:149-52, 155-6.
47. Podoshin L, Fradis M, Malatskey S, Ben-David J. Type I Tympanoplasty in Children. Am J Otol 1996; 17:293-6.
48. Stenfors LE, Carlsöö B,Winbland B. Repair of Experimental Tympanic Membrane Perforations. Acta Otolaryngol 1980; 90:332-41.
49. Mondain M, Ryan A. Effect of Basic Fibroblast Growth Factor on Normal Tympanic Membrane. Am J Otolaryngol 1994; 15:344-50.
50. Oliveira, JAA, Bernal TMO. Fatores neurotróficos de crescimento. In: Campos CAH, Costa HOO. Tratado de Otorrinolaringologia. 1ª Edição. São Paulo: Editora Roca; 2003. v. 1, p. 138-55.
1 Faculty Professor, Department of Ophthalmology, Otorhinolaryngology and Head and Neck Surgery, Medical School, Ribeirão Preto - University of São Paulo - USP.
2 Assistant physician, Hospital das Clínicas, Medical School, Ribeirão Preto - USP.
3 Associate Professor, Department of Biochemistry and Immunology, Medical School, Ribeirão Preto - USP.
4 Post-graduation under course, Department of Surgery, Medical School, Ribeirão Preto - USP.
Department of Ophthalmology, Otorhinolaryngology and Head and Neck Surgery, Medical School, Ribeirão Preto - USP -
Area of Otorhinolaryngology, (Otology) - Hospital das Clínicas de Ribeirão Preto.
Article submitted on April 14, 2003. Article accepted on July 24, 2003.