Year: 2003 Vol. 69 Ed. 4 - (7º)
Artigo Original
Pages: 480 to 483
Surgical treatment of the squamous cell carcinoma of tongue and floor of mouth: pos-operative radiotherapy efect
Author(s):
Ali Amar1,
Otávio Alberto Curioni1,
Sérgio T. Higuchi2,
Abrão Rapoport1
Keywords: squamous cell carcinoma, tongue, floor mouth, metastases, lymph node, radiotherapy
Abstract:
Assessment of the postoperative irradiation effectiveness for improvement local control of squamous cell carcinoma of tongue and floor of the mouth in stages III and IV. Study Design: clinical retrospective. Material and Method: a retrospective case-series of 72 patients with squamous cell carcinoma of tongue and floor of mouth who underwent surgery. The local control rate was assessed in front of stage, surgical margins and postoperative radiotherapy. Results: local recurrences were diagnosed in 28 (38%) patients. Only T stage and lymph node metastases were related to recurrences in the primary site. Control of disease in the primary site was achieved in 80% of pN0 patients and postoperative radiotherapy didn't show any effect in this group. Conclusion: advanced local disease without lymph node metastases can be treated with surgery only.
![]()
INTRODUCTION
Combined therapy of surgery and postoperative radiotherapy is the established standard treatment of advanced squamous cell carcinoma of aerodigestive tract. The advanced stage of the disease is defined by the presence of extensive primary tumor (larger than 4 cm in its greater diameter or infiltration of adjacent structures), and the presence of local and distant metastases indicates the configuration of clinical stage III or IV.
Surgical treatment of squamous cell carcinoma of the tongue and floor of the mouth at locally advanced stage (T3 and T4) includes neck dissection. It is recommended even in the absence of clinically identifiable metastases as elective dissection, since blind metastases rate is higher than 30%1. Postoperative irradiation treatment generally includes primary site and both sides of the neck, regardless of its recommendation being related to the primary site or to metastases. If anatomical and pathological analysis confirms the absence of lymphatic metastases (pN0), the neck will not have to receive additional treatment 2. Conclusive studies related to the benefits of postoperative treatment of squamous cell carcinoma of the tongue have not been conducted yet. According to Zelefski et al.3, tongue carcinomas present high rates of local recurrence regardless of postoperative radiotherapy. Most of the patients within stage T3 and T4 have lymph node metastases that can justify the indication for postoperative irradiation.2,4. This therapy, however, is arguable for those patients who had their tumor resected with free margins 5.
The objective of the present study was to evaluate the treatment results of extensive tongue and floor of the mouth tumors considering the effect of postoperative radiation therapy against local control of the disease.
MATERIAL AND METHOD
Clinical records of patients with squamous cell carcinoma of tongue and floor of the mouth with primary stage of T3 and T4 (UICC-AJCC, 1997) that had undergone surgery in the Department of Head and Neck Surgery and Otorhinolaryngology, Hospital Heliópolis, Sao Paulo, from January 1978 to December 1998 were reviewed. The study included 113 patients that had not received treatment. Forty-one patients were excluded with no evidence of local recurrence of the disease with follow-up shorter than 18 months. Among seventy-two of the remaining patients, sixty-four were men and eight were women. Mean age was 54 years (from 35 to 84 years). All patients underwent neck dissection associated with primary tumor resection and thirty-seven (51%) received external postoperative radiation with doses ranging from 5000 to 7000 cGy (average dose of 5500 cGy).
Disease control rate in the primary site was assessed considering the performance of postoperative radiation. Additionally, evaluation considered primary tumor staging (T), presence of lymph node metastases in clinical pathology analysis carried out after neck dissection, (pN) and the condition of the resected margin of the primary tumor (free or involved). Margin evaluation was carried out with optical microscopy and they were considered involved when the limit of the tumor matched resected limits and minimal distances between the tumor and the resection limit were below 5 mm. Involved margins or exiguous margins were jointly considered.
Statistical analysis applied Fisher's accuracy test for qualitative variables. Multivariate analysis applied the logistic regression method, including significant variables in the univariate analysis and performance of postoperative radiation. Accepted differences had p value d<0.05.
RESULTS
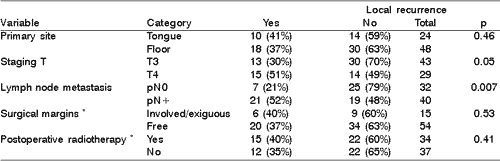
Local recurrence was diagnosed in 28 (38%) patients. In the analyzed variables only T stage and presence of histological positive lymph node metastases showed association with primary site recurrence (Table 1).
Nine out of 15 patients with involved surgical or exiguous surgical margins underwent postoperative radiation. Recurrence in the primary site in this group was found in three irradiated patients (33%) and in three non-irradiated patients (50%), p=0.62.
At the moment of the initial treatment, disease was limited to primary site in 32 patients (pN0). Recurrence in the primary site was found in 5 (20%) out of 24 non-irradiated patients and in 2 (25%) out of 8 irradiated patients in the postoperative period (p=0.57).
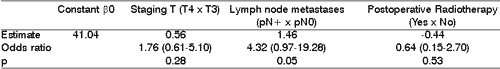
In multivariate analysis only the presence of lymph node metastases showed some relation to recurrence in the primary site. Postoperative radiation decreased local recurrence of the disease, however it was not statically significant (Table 2).Table 1. Local recurrence versus primary site, staging T, presence of lymphatic metastases, surgical margins and postoperative radiotherapy - Univariate analysis.
* information not available about all patients.
pN0 = absence of lymph node metastases in the clinical pathology analysis
pN+ = presence of metastases in one or more lymph nodes in the clinical pathology analysis
Table 2. Local recurrence versus primary site, staging T, presence of lymphatic metastases, surgical margins and postoperative radiotherapy - multivariate analysis.
DISCUSSION
Extensive lesions of the tongue frequently affect floor of the mouth and vice-versa. Although a distinct behavior is expected among those tumors of these two secondary sites, the need to increase sampling size of studies makes them to be grouped as the same disease.6. Likewise, a T4 tumor does not necessarily represent the growth of a T3 tumor. T4 staging is characterized by the invasion of the mandible (except for the invasion restricted to the alveolar process) or of the extrinsic muscles of the tongue, and it reflects different biological behavior. Higher risk of local T4 tumors against T3 tumors could be related to these functional characteristics and not to the size of the tumor. Some variables associated with metastatic disease with tumor differentiation, perineural infiltration, vascular or lymph node embolization could be related to recurrence in the primary site, and however they were not considered in this study 1. Functional evaluation, taking into account factors such as angiogenesis, integrin expression and MMPA production, could supply additional relevant date related to the tumor, enabling increased understanding of outcome and natural course of the disease7.
The association between lymph node metastases and higher recurrence risk in primary site has already been reported 8. This result suggests not only higher likelihood of residual microscopic disease after resection of such tumors, but also increased thriving capacity of these residual cells. Considering this hypothesis, some cases of controlled disease could also present some quiescent tumor focus.
Resection of tongue lesions commonly present involved margins more frequently than in other sites of the oral cavity. 9 Although surgical margins receive a lot of attention of the surgeons, they are not reliable for assessing the risk of local recurrence. Notwithstanding the fact that tumor was not found in 73% of the procedures of magnification of involved margins, misdiagnosis of free margin also may occur10. Many patients with involved resected margins did not have local recurrence, in spite of not receiving additional treatment, whereas patients with free margins presented recurrence regardless of receiving combined therapy. Moreover, there is the possibility that some local recurrences are second tumors related to field cancerization11. Recommendations for extensive lesions include intraoperative assessment with frosting exam of the margins, since magnification in a second time presents some technical difficulties and postoperative radiation does not ensure local control in such cases. This is the key principle of oncological surgery; it is not enough, however, to define individual outcome or reference for additional treatment.
In this study, therapeutic effect of postoperative radiation was probably masked by patient selection with more advanced stages of disease. Although combined therapy has been advocated for advanced stage diseases, results are far from those expected by the sum of individual results. Certainly, some patients do not benefit or some can even be negatively affected by this therapeutic approach. Patients with extensive primary tumors of the tongue and floor of the mouth, in the absence of lymph node metastases, presented control of the disease in approximately 80% of the cases treated only with surgery, and this makes the indication of postoperative radiation questionable for this group. Other authors have also reported similar local control rates 12. Considering that the incidence of a second primary tumor in patients with squamous cell carcinoma of upper airways and digestive tract is approximately 4% a year and that many of those second tumors occur again in the oral cavity, it could be more adequate to reserve this therapeutic tool to treat second or recurrent cases since previous radiation therapy does not allow new irradiation in effective doses13. Postoperative radiation could be considered after reconstruction, which makes it difficult to have early diagnosis of recurrence, or when initial resection limits make a salvage surgery unlikely, especially in cases that present extensive involvement of suprahyoid muscles. In cases with no local metastases (pN0), there is no reason to include infrahyoid structure in the irradiation field once neck dissection was performed. The benefit of postoperative irradiation regarding oncological control should also be weighted against sequels such as xerostomia, irradiation cavities, and osteoradionecrosis of mandible and cervical fibrosis, which have negative impact on quality of life of the patients. These aspects became more important in therapeutic plans, especially because more aggressive therapeutic approaches did not significantly modify survival rate of these patients 14.
The effect of postoperative radiation should be assessed in each anatomical site, through prospective randomized-controlled studies and it requires large number of patients and multicentered studies. Postoperative recommendation should be individualized, once the patients with a disease limited to the primary site (tongue and floor of the mouth) present high rates of local control after surgical treatment alone.
REFERENCES
1. Kowalski LP & Medina JE. Nodal metastases: predictive factors. Otolaryngol Clin North Am 1998;31:621-637.
2. Brugère JM, Mosseri VF, Mamelle G. Nodal failures in patients with N0N+ oral squamous cell carcinoma without capsular rupture. Head Neck 1996;18:133-7.
3. Zelefsky MJ, Harrison LB, Fass DE. Postoperative radiation therapy for squamous cell carcinomas of the oral cavity and oropharynx: impact of therapy on patients with positive surgical margins. Int J Radiation Oncology Biol Phys 1993;25:17-21.
4. Smeele LE, Leemans CR, Langendijk JA. Positive surgical margins in neck dissection specimens in patients with head and neck squamous cell carcinoma and the effect of radiotherapy. Head Neck 2000;22:559-63.
5. Gonzales-Botas JH, Barro CV, Amado ML. Factors affecting local and regional control and survival of carcinomas of the tongue and floor of mouth. Acta Otorrinolaringol Esp 2002;53:32-8.
6. Zelefsky MJ, Harrison LB, Fass DE. Postoperative radiotherapy for oral cavity cancers: impact of anatomic subsite on treatment outcome. Head Neck 1990;12:470-475.
7. Yorioka CW, Coletta RD, Alves F. Matrix metalloproteinase-2 and -9 activities correlate with the disease-free survival of oral squamous cell carcinoma patients. Int J Oncol 2002;20:189-94.
8. Leemans CR, Tiwari R, Nauta JJ. Recurrence at the primary site in head and neck cancer and the significance of neck lymph node metastases as a prognostic factor. Cancer 1994;73:187-190.
9. Jones AS, Bin Hanafi Z, Nadapalan V. Do positive ressection margins after ablative surgery for head and neck cancer adversely affect prognosis? A study of 352 patients with recurrent carcinoma following radiotherapy treated by salvage surgery. Br J Cancer 1996;74:128-132.
10. Lee JG. Detection of residual carcinoma of the oral cavity, oropharynx, hypopharynx, and larynx: A study of surgical margins. 78th Annual Meeting of the American Academy of Ophthalmology and Otolaryngology, Dallas, 1973.
11. Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium. Cancer 1953; 6: 963-968.
12. Loree TR & Strong EW. Significance of positive margins in oral cavity squamous carcinoma. Am J Surg 1990;160:410-414.
13. León X, Quer M, Diez S. Second neoplasm in patients with head and neck cancer. Head Neck 1999;221:204-210.
14. DeStefani A, Magnano M, Cavalot A. Adjuvant radiotherapy influences the survival of patients with squamous carcinoma of the head and neck who have poor prognoses. Otolaryngol Head Neck Surg 2000;123:630-6.
1 Surgeon, Department of Head and Neck Surgery and Otorhinolaryngology, Hospital Heliópolis, Hosphel, Sao Paulo, Brazil.
2 Resident Physician, Department of Head and Neck Surgery and Otorhinolaryngology, Hospital Heliópolis, Hosphel, Sao Paulo, Brazil.
Address correspondence to: Dr. Ali Amar - Rua Pintassilgo 516 ap.188
V. Uberabinha São Paulo SP 04514-032 - E-mail: lalitos@terra.com.br
Study conducted at the Department of Head and Neck Surgery and Otorhinolaryngology, Hospital Heliópolis, Hosphel, Sao Paulo, Brazil.
Article submitted on September 11, 2002. Article accepted on March 13, 2003.