Year: 2001 Vol. 67 Ed. 5 - (11º)
Artigos Originais
Pages: 677 to 681
Total salivary non stimulated flow evaluation, after radiotherapy for patients with mouth and oropharynx squamous cell carcinoma with the association of lauryl-dietylene-glycol-ether sodium sulphate and calcium hydroxide
Author(s):
Christiano M. Correia 1,
Marco A. O. Marinho 1,
Abrão Rapoport 2,
Eurípedes O. Marinho 3,
Neil F. Novo 4,
Iara Juliano 4
Keywords: salivary flow, mouth neoplasia, sodium dodecilsulfate, calcium hydroxide
Abstract:
Introduction: Evaluation of quantitative non-stimulated salivary flow rate in 11 patients with oral and oropharyngeal squamous cell carcinoma after exclusive radiation therapy, treated with the association of lauryl-diethylene-glycol-ether-sodium sulfate and calcium hydroxide. Study design: Prospective clinical no randomized. Method: salivary flow was evaluated in three phases: 1) during seven weeks when they were submitted to radiotherapy with total dose of 7,000 cGy, without salivary stimulation with the association of drugs; 2) during eight weeks after radiation treatment, when the patients had salivary stimulation with the association of drugs; 3) for eight weeks after the end of treatment with the association of drugs. Results: These data were analysed in order to evaluate the efficacy of the medicament in avoiding salivary hypofunction after radiotherapy. Ten out of eleven patients showed significant better outcome in the salivary flow rates with the use of drugs related to the values obtained at the final period of radiotherapy and mantained these rates even without continuing using the drugs. Conclusions: the association of drugs was effective in the treatment of salivary hypofunction and promoted increase, in the total non-stimulated salivary flow rates in ten out of eleven patients, and this enhancement, was maintained for two months after interruption of the use of the medicament.
![]()
INTRODUCTION
In Brazil, diagnosis of mouth cancer is normally made in stages III and IV of the disease. Eight percent is detected in male patients and 2% in female patients, characterizing a 4:1 ratio and representing the 6th position among the most frequent malignant neoplasia15. For the treatment of neoplasia (90% of squamous cell carcinoma) the multidisciplinary focus (surgery, radiotherapy and chemotherapy) replaced isolated therapies in recent decades21. The use of radiotherapy in mouth and oropharynx advanced cancer became a rule practiced by different forms of therapeutic association, leading to undesirable side effects. Among them, acute effects may lead to secondary cell death, resulting in incapacity to recover DNA and lesions of organelles caused by the action of ionizing radiation and production of intracellular free radicals5,7,12,14. As a result of molecular damage, irradiated tissues show at microscopy hyalinization and occlusion of small vessels6, replacement of glandular acini by loose collagen connective tissue11,14,25,26. Consequently, salivary hypofunction leads to subjective perception of dry mouth, named xerostomia8,9,18, reaching up to 93% of salivary flow decrease4.
Therefore, in order to protect the oral cavity of the patients during radiation, we tried to employ lauryl-diethylene-glycol-ether-sodium sulfate, check the action of this association in the prevention of radiation treatment effects, through the increase of salivary flow, and compare it to placebo2,16,20.
Based on these findings, we quantitatively evaluate the mean of non-stimulated total salivary flow in patients with squamous cell carcinoma of the mouth and oropharynx, after radiotherapy treatment only, treated with the association of lauryl-diethylene-glycol-ether-sodium sulfate and calcium hydroxide. This association acts as an antibacterial plaque agent19, leading to non-stimulated total salivary flow increase above the baseline level of salivation16.
MATERIAL AND METHOD
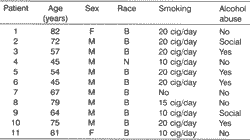
At Hospital Dr. Helio Angotti, from Associação de Combate ao Câncer do Brasil Central, Uberaba/MG, from March 1999 to October 2000, we studied 11 patients who had mouth and, oropharynx squamous cell carcinoma . The mean age was 65.5 years, divided into 9 men and 2 women and four were alcohol abusers and 10 were smokers (Table 1).
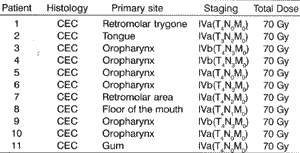
As to staging, they had stage IV carcinomas. (UICC, 1998), seven in IVa stage and four in IVb, all irradiated with a total dose of 7,000Gy (Table 2), at daily doses of 200cGy, in 5 weekly sessions for 7 weeks.Table 1. Personal data of studied patients.
M = Male; F = Female; B = Caucasian; N = Black; Cig = Cigarettes
Table 2. Relation of patients according to histology type, primary site, staging and total irradiation dose.
CEC = Carcinoma espinocelular; Gy = Gray
As to non-stimulated total saliva flow, the collection was made with seated patients, head at 90° the saliva was expelled through the mouth in a lab tube, in three different stages with 5-minute intervals, as proposed by Dawes (1987)1:
1st stage: collection (C0) without the use of medication before the beginning of radiation - baseline salivation and seven collections during radiotherapy (C1, C2, C3, C4, C5, C6 and C7);
2nd stage: six collections making use of drugs, for 8 weeks after radiotherapy (C8 to C11 with the use of HcT20 in the first month, and C12 and C13 in the second month, after use of HcT20).
3rd stage: two collections in 12 and 16 weeks after radiation without use of medication (C14 and C15 after suspension of HcT20).
At the end of each collection, we added 3ml of distilled water to let the residue flow from the walls of the funnel.
As to the used medication, we recommended the association called HcT20: 800 ml distilled water, 200ml lauryl-diethylene-glycol-ether-sodium sulfate (0.125g/ml) and 2g calcium hydroxide. We adopted 0.1ml/min as minimum value of non-stimulated salivary flow to define salivary hypofunction. The use of medication was administered as two daily mouthwashes with l0ml solution (first the bottle was shaken to enable mix of the solute) in the morning and at night.
The statistical analysis of the 16 collections were made by Friedman's variance24 for each patient, studying the values of non-stimulated total salivary flow observed in different periods. The same analysis was employed to compare the percentage differences (? %) between nonstimulated total salivary flow observed at the end of radiotherapy, with the use of HcT20 (? %1) during radiotherapy, with its use (? %2) after radiotherapy and without its use (? %3) in the period, using as a formula:
? %=(collection value Ci - collection value CO)/collection value CO x 100
CO is the baseline collection and Ci is in the various periods. If there were statistically significant differences, Friedman's analysis was complemented by multiple comparison tests13. In all tests, we defined 0.05 or 5% as rejection level of nullity of hypothesis, and significant results were marked with asterisks.
RESULTS
All patients in the studied group presented gradual reduction of the non-stimulated total salivary flow at the end of radiotherapy treatment, compared to the initial collection (CO).
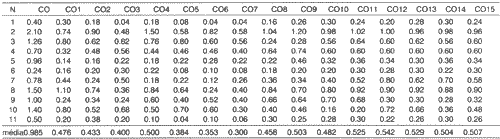
Three patients 1, 6 and 11 presented marked salivary hypofunction of non-stimulated total salivary flow with values below 0.1ml/min at the end of radiotherapy, with values of 0.04, 0.08 and 0.06ml/m, respectively. Eight weeks after treatment with HcT20 (C15), the patients presented values of 0.24, 0.30 and 0.26m1/min, respectively. In the first collection, after one week of HcT20 use (C8), all patients, except for patients 3 and 5, had an increase of non-stimulated total salivary flow compared to the end of radiotherapy (C7). After 4 weeks of treatment (C11), only patient 10 presented reduction of non-stimulated total salivary flow compared to C7. Values of collections C12 and C13 (six and eight weeks of use of medication) demonstrated that 10 in 11 patients had increase of nonstimulated total salivary flow compared to C7. Values of C14 and C15, equivalent to 4 and 8 weeks after interruption of HcT20 use, showed that non-stimulated total salivary flow levels were stable compared to C13 and higher than C7 levels, except for patient 9. The values of non-stimulated total salivary flow collection before radiotherapy (C0), during radiotherapy (C1 to C7), after radiotherapy with the use of medication (C8 to C13), and after the interruption of the drug (C14 and C15), are expressed in Table 3.
At the end of radiotherapy, all patients complained of xerostomia, and saliva became thicker and sticky. The mean values for C0, C7, C13 and C15 in ml/min were respectively, 0.985; 0.300, 0.529 and 0.507.
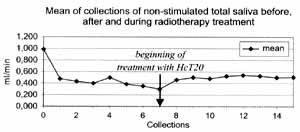
The mean of weekly collections of non-stimulated total saliva are shown in Table 3 and Figure 1.
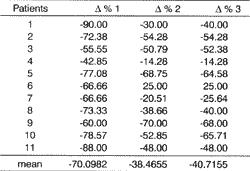
Based on the results of Table 3, we calculated the percentage differences of salivary flows observed in C7, C13 and C15. These data are presented in Table 4 and Figure 2.Table 3. Values of non-stimulated total saliva collections in studied patients before, during and after radiotherapy.
Friedman's analysis of variance
(X2)r calculated =37.47*
(X2) critical=23.68 (P<0.001)
Test of multiple comparisons
Collection 11 >Collection 5 and 6
Collection 9, 14 a 15> Collection 7
N = n of patients
CO = baseline salivation
C1 to C7 = during RxT
C8 a C13 = post-RxT with medication
C14 a C15 = post-RxT without medication
Figure 1. Graph of means of salivary flow obtained from Table 3.
Percentage reduction of salivary flow observed between periods C0 and C7 (? %1) was significantly greater than that observed for periods C0 and C13 (? %2) and CO and C15 (? %3), which did not differ one from the other.
DISCUSSION
The search for better quality of life for patients with squamous cell carcinoma of the mouth and oropharynx necessarily includes the development of techniques and drugs that minimize the reduction of salivary flow resultant from radiotherapy, represented by xerostomia as the most important undesirable effect7,10,27. The irradiate salivary gland presents degenerated alterations, inflammations, endarteritis, thrombosis, hyalinization and glandular atrophy11,22. Such alterations are dose-dependent, the degree of xerostomia is a result of the amount of irradiated tissue, and permanent salivary hypofunction happens at 3,500 to 6,000 cGy23.Table 4. Percentage difference (? %) of salivary flow observed in saliva collections C7 (? % 1), C13 (? % 2) and C15 (? % 3).
Friedman's variance analysis
(X²) calc=12.60* (p < 0.01)
(X²)crit=5.99
? %1 < ? %2 and ? %3
In the present study (Table 3), we noticed that patients who had salivary flow rates greater than 0.96m1/ min before radiotherapy were those that had reduced levels of xerostomia (patients 2, 3, 5, 8, 9 and 10), which is in accordance with the literature.
We set a minimum level of non-stimulated total saliva of 0.1ml/min and, in our sample, three patients (1, 6 and 11) presented at the end of radiotherapy (C7) levels of 0.04, 0.08 and 0.06 ml/min, with good responses after the use of HcT20, with levels of 0.24, 0.30 and 0.20 ml/min, respectively. Patient 2, with baseline salivary flow (CO) of 2.10 ml/min, at the end of HcT20 after the radiotherapy period presented flow of 0.96 ml/min, a stable amount two months after suspension of the medication. Patients 3, 8, 9 and 10 had baseline flow (CO) of 1.26, 1.50, 1.40 and 1.OOml/min and in C13, of 0.60, 0.90, 0.23 and 0.48 ml/min. Finally, patients 4, 5 and 7, had CO of 0.70, 0.96 and 0.78 ml/min, in C13 they presented 0.60, 0.30 and 0.62 ml/min and in C15, 0,60, 0,34 and 0.58 ml/min, respectively.
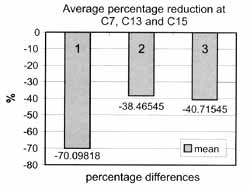
It shows mean values of 0.98 ml/m in C0, 0.30 at the end of radiotherapy and 0.52 after use of HcT20 in the post-radiation period and 0.50 when HcT20 was suspended, being that at the end of radiotherapy all patients referred xerostomia. After the use of HcT20 (Figure 1), we showed that values tended to become stabilized (from C7 to C14). In the study of percentage differences of C7 (end of radiotherapy), C13 (end of HcT20 use) and C15 (end of observation period without HcT20), we obtained values of -70.0982, -38.4655, and -40.7155, respectively (Table 4 and Figure 2), observing that the value of 0%2 (period of use of HcT20) and A%3 (period of observation after suspension of HcT20) did not differ one from the other.
Figure 2. Percentage differences (D%) of slivary flows observed in saliva collections C7 (D%1), C13 (D%2) and (D%3).
After its historical use an oral antiseptic agent effective to reduce the bacterial plaque3,19, HcT20 started to be used as a medicantion17,23,24 to prevent cases of salivary hypofunction in patients submitted to oral radiotherapy. Some patients complained of reduction of salivary flow with 3,000Gy and at 6,000Gy there is up to 70% reduction of salivary flow, according to the literature16,20. By using HcT20 for 8 weeks after radiotherapy, there was a mean increase of salivary flow of 32% among our 11 patients, which amounts to half of the mean value obtained before radiotherapy.
Therefore, we observed mean values of salivary flow of 0.98m1/min before radiotherapy, 0.47ml/min after one week and 0.30ml/min at the end of radiotherapy. However, at the end of treatment with HcT20, the mean was 0.52ml/min and in 8 weeks, without its use, the mean was 0.50ml/min.
CONCLUSIONS
To sum up, in our experience, salivary flow at the end of the study (C15) was higher than at the end of radiotherapy (C7), and, in average, half of the baseline value (C0) in 10 out of 11 patients. We concluded that there was a salivary flow increase in the group of patients who were treated with the medication.
REFERENCES
1. BARBOSA, S.V. - Atividade antimicrobiana da solução de hidróxido de cálcio pura a em associação com detergente sobre microorganismos dos canais radiculares. Análise "in vitro". Natal, 1984. 150p Tese Mestrado - Universidade Federal do Rio Grande do Norte.
2. BARBOSA, S.V.; ALMEIDA, D. - HCT20 - Uma solução irrigadora para canais radiculares humanos. Análise "in vitro". Rev. Bras. Odont., 44(5):21-8; 1987.
3. BARBOSA, S.V.; SEABRA, E.J.G.; SEABRA, E.G. - Redução da placa bacteriana com o uso de clorexidina a HcT 20 em pacientes com gengivite a periodontite crônicas. Robrac, 9(5):13-7; 2000.
4. BEUMER, J.; CURTIS, T.; HARRISON, R.E. - Radiation therapy of the oral cavity: sequelae and management, part 1. Head Neck Surg., 1(4):301-12; 1979.
5. BOTTOMLEY, WK.; EBERSOLE, J.H. - Guidelines for dental care when patients receive radiation therapy to the head and neck. Oral Surg. Oral Med. Oral Pathol., 22(2):252-6; 1966.
6. BROWN, L.R.; DREIZEN, N.S.; HANDLER, S.; JOHNSTON, D.A. - Effect of radiation-induced xerostomia on human oral microflora. J. Lent. Res., 54(4):740-9; 1975.
7. COLEMAN III, J J. - Management of radiation-induced soft tissue injury to the head and neck. 1. Dent. Res.. 20(3):491-505: 1993.
8. DAVIES, A.N.; SINGER, J. - A comparison of artificial saliva and pilocarpine in radiation-induced xerostomia. J. Laryngol. Otol., 108:663-5; 1994.
9. DREIZEN, S.; BROWN, L.R.; DREIZEN, S.; HANDLER, S.; JOHNSTON, D.A. - Radiation-induced xerostomia in cancer patients. Effect on salivary and serum electrolytes. Cancer, 38(7): 273-8; 1976.
10. EPSTEIN, J.B.; EMERTON, S.; LE, N.D.; STEVENSON-MOORE, P. - A double -blind crossover trial of Oral Balance gel and Biotene(r) toothpaste versus placebo in patients with xerostomia following radiation therapy. Oral Oncology, 35(1):132-7; 1999.
11. FISHER, J.C. - Introduction to radiation injury. Clin. Plast. Surg., 20(3):431-3; 1993.
12. FLETCHER, G.H. - Textbook of radiotherapy. 3.ed. Philadelphia: Lea Febieger; 1980.
13. HOLLANDER, M.; WOLFE, D.A. - Nonparametric statistical methods. New York: John Wiley & Sons - 503 p.; 1973.
14. KASHIMA, H.K.; KIRKHAM, W.R.; ANDREWS, J.R. - Post-irradiation sialadenitis. A study of the clinical features, histopathologic changes and serum enzyme variations following irradiation of human salivary glands. Am. J. Roentgenol., 94(2):271-91; 1965.
15. KOWALSKI, L.P.; NISHIMOTO, I.N. - Epidemiologia do câncer de boca. In: PARISE Jr., O. Câncer de Boca - aspectos básicos e terapêuticos. São Paulo: Sarvier, 256(1):3-11; 2000.
16. MARINHO, M.A.O. - Avaliação do fluxo salivar durante radioterapia com o uso da associação de laud-dietfeno-glicol-titer-sulfato de sódio e hidróxido de cálcio. Tese - Mestrado - Complexo Hospitalar Heliópolis. São Paulo, 96p; 1999. .
17. MAKKONEN, T.A.; NORDMAN, E. - Estimation of long-term salivary gland damage induced by radiotherapy. Acta Oncol., 26-307; 1987.
18. NEDERFORS, T.; ISAKSSON, R.; MORNSTAD, H.; DAHLF, C. Prevalence of perceived symptoms of dry mouth in an adult Swedish population - relation to age, sex and pharmacotherapy. Community Dent. Oral Epidemiol., 25(3):211-6; 1997.
19. PIMENTA, F.H.; BARBOSA, S.V. - A eficácia do HCT20 como agente antiplaca. Rev. ABO Nac., 6(1):33-6; 1998.
20. RODOR, R.C. - Proteção de cavidade oral com HcT20 em pacientes irradiados. Monografia para especialização-ABO. Brasília, 84p.; 1988.
21. RUBIN, P. - Manual de Clínica Oncológica. 4 ed., São Paulo: Sarvier; 1977.
22. SCHULLER, D.E.; STEVENS, P.; CLAUSEN, K.P.; OLSEN, J.; GAHBAUER, R.; MARTIN, M. - Treatment of radiation side effects with oral pilocarpine. J. Surg. Oncol., 42:272-6; 1989.
23. SENAHAYAKE, F.; PIGGOTT, K.; HAMILTON-MILLER, J.M.T. - A Pilot Study of Salix SST (Saliva-stimulating Lozenges) in post-irradiation Xerostomia. Carr Med Res and Opin, 14(3):155- 9; 1998.
24. SIEGEL, S.; CASTELLAN Jr, NJ. - Nonparametric Statistics. 2. ed., New York, McGraw-Hill Int. 399p., 1988.
25. WECKX, L.L.M.; RODRIGUES, E. - Hipossalivação. Rev. Bras. Med., 4 7(7):283-8; 1990.
26. WESCOTT, W.B.; MIRA, J.G.; STARCKE, E.N.; SHANNON, I.; THORNBY, J.I. - Alterations in whole saliva flow rate induced by fractionated radiotherapy. Am. J Roentgenol., 130:145-9; 1978.
27. WHITMYER, C.C.; WASCOWSKI, J.C.; IFFLAND, H.A. - Radiotherapy and oral sequelae: preventive and management protocols. J. Lent. Hyg., 71(1):23-9, 1997.
1 Master in Head and Neck Surgery, Hospital Heliópolis, São Paulo, SP.
1 Coordinator of Post-Graduation Course, Hospital Heliópolis, São Paulo, SP.
3 Ph.D. in Surgery, FMUSP, Ribeirão Preto, SP.
4 Ph.D. in Preventive Medicine, UNIFESP, São Paulo, SP.
Study conducted at Hospital Dr. Hélio Angotti, Associação de Combate ao Câncer do Brasil Central - Uberaba, MG.
Address correspondence to: Prof. Dr. Abrão Rapoport - Praça Amadeu Amaral, 47 - cj. 82 - Paraiso - CEP - 01327-010 - São Paulo - SP - Telefax (55 11) 273-8224 - E-mail: cpgcp.hosphel@attglobal.net
Article submitted on April 20, 2001. Article accepted on May 30, 2001.