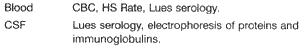Year: 2001 Vol. 67 Ed. 5 - (5º)
Artigos Originais
Pages: 636 to 642
Auditory neuropathy: relevant aspects to clinical investigation
Author(s):
Osmar Mesquita Neto 1,
Maria C. Redondo 2,
Rosimeire C. Carlos 3,
Marina S. Figueiredo 4,
Otacílio C. Lopes Filho 5
Keywords: clinic, neuropathy, auditive
Abstract:
Introduction: The term auditory neuropathy defines a group of alterations based on the results of supplementary hearing tests. Aim: To observe the occurrence of hearing signs and symptoms in subjects with auditory neuropathy. Study design: Retrospective clinical. Material and Method: Analysis of 30 cases of auditory neuropathy diagnosed between June 1995 and June 1997, whose onset of symptoms took place after speech acquisition. We emphasized history, physical exam, supplementary tests and audiological assessment. Results: This series of patients showed the prevalence of various signs and symptoms peculiarly associated. Conclusion: There are a number of signs and symptoms, regardless of being associated or not, that may suggest the presence of auditory neuropathy.q
![]()
INTRODUCTION
As from 1995, we started to hear the term auditory neuropathy14, relating to alterations characterized by falling to record brainstem auditory evoked potentials and presence of otoacoustic emissions within normal ranges1. However, this characterization lacks detailing of clinical picture; sometimes it is described as excessive difficulty comparing to speech understanding associated with mild to moderate hearing loss in the audiogram2,7,17.
The description of cases with similar hearing deficit may be found in previous case reports6,8,11,13, normally associated with (clinical) well-established neurological cases. It is very likely that at least some of these patients would be diagnosed as having auditory neuropathy, based on current instruments and concepts of hearing assessment.
Jerger10 commented on the fact that since there is a number of patients with neurological disorders of the hearing, some selected cases require specific tests; however, he never said what the criteria for selection should be.
The diagnosis of auditory neuropathy is a result of the application of current assessment tools to subjects who have specific hearing disorders (or to children who belong to some kind of risk group)1,4,5,12.
The purpose of the present article was to analyze the results of anamnesis, physical examination and conventional hearing assessment (pure tone audiometry, speech discrimination and immitance measures) in 30 patients with auditory neuropathy diagnosed through audiological assessment and distortion product otoacoustic emissions (DPOAEs), plus brainstem auditory evoked potentials.
MATERIAL AND METHOD
We evaluated 30 patients diagnosed with auditory neuropathy, whose results of brainstem auditory evoked potentials were absence or partially present and incompatible with the presence of DPOAEs amplitudes within or greater than the normal range. There were 13 female and 17 male subjects ranging in age from 11 to 61 years (mean age of 32.95 years), treated from June 1995 to June 1997 at the Department of Otorhinolaryngology, Santa Casa de São Paulo.
All patients included in the present study were assessed and studied by the same examiner.
History data are presented in Chart 1.
Laboratory tests consisted of serum levels and cerebrospinal fluid (CSF) analysis, described in Chart 2.
All patients (30) were submitted to computed tomography and 10 of them to magnetic resonance imaging (at that time, our hospital did not have the device available; therefore, we would like to thank the support of Hospital do Coração and Flávio Madureira Padula, MD, for having received our 10 patients).Chart 1. Studied data from the anamnesis.
Chart 2. Laboratory tests performed.
Basic audiological assessment consisted on pure tone audiometry, speech tests (speech recognition index and threshold), tympanometry and contralateral stapedial muscle analysis.
We also performed brainstem auditory evoked potentials and distortion product otoacoustic emissions.
RESULTS
Anamnesis and physical examination
All 30 patients reported hearing problems, characterized by difficulty to understand speech. Despite the fact that other types of hearing disorders rather than neuropathy could have caused the same symptom, this finding was consistent and should be valued.
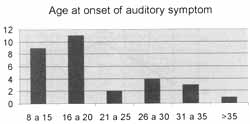
The age at onset of symptoms varied from 8 to 38 years, mean age of 18.6 years (Graph 1).
Symptoms were normally noticed during the two first decades of life.
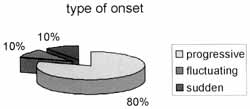
The type of onset was described as progressive by 24 patients; fluctuating by 3, and sudden by other 3 patients (Graph 2). In the case of progressive and fluctuation onset, patients reported that the complaint was stabilized after some weeks to three months, but no patients reported current worsening of hearing complaint.
The interval from the onset of hearing complaint and the last medical visit varied from 1 to 35 years, mean of 11.36 years.
The presence of tinnitus was referred by 28 patients. It was defined as low frequency by 24 patients, high frequency by two patients and poorly defined by two other patients (Graph 3). The noisy environment was described as a driver of tinnitus in 19 patients (Graph 3).
Only three patients complained of occasional mild vertigo.
Graph 1. Age at onset of hearing complaint.
Hearing aids had been used by 16 patients. In no case was there description of improvement in social performance as a result of its use. Hearing aids try to imitate the amplifying characteristics of the cochlea, especially that of the outer hair cells. The fact that no one benefited from its use made the examiner wonder whether the sound amplification mechanisms of the body were intact (middle ear, outer hair cells) and if it was a valid therapeutic approach.
Only 4 patients reported similar cases in the family, and two of them were the same family (mother and son).
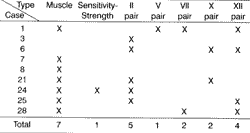
Symptoms and/or neurological alterations were identified in nine patients (Chart 3).
The presence of non-hearing neurological alterations was not correlated with family history. Non-hearing neurological symptoms started 6 to 36 months after the onset of the hearing complaint (mean of 18 months).
Three patients presented clinical picture compatible with hereditary sensorial-motor neuropathy (Charcot-Marie-Tooth disease), including reduction of neural conduction rate at electromyography. Although there was no family history, the hypothesis was maintained until the performance of a genetic study.
Perceptual voice analysis was conducted in 16 cases. Such a procedure showed very interesting results that will be thoroughly described in another study.
Serum tests did not reveal alterations in any patient. Analysis of CSF was abnormal in one case, with elevation of protein level slightly above the maximum normal threshold.
Head and brainstem CT scan showed alteration of the corpus callosum in one patient, with reduction of volume, suggesting atrophy (the MRI showed the same abnormality). This alteration is not directly related with hearing impairment. No abnormalities were detected in the imaging diagnosis of the other 29 patients (29 CT scans and 9 MRI studies).
Pure tone audiometry and speech recognition index
The thirty patients presented abnormal hearing thresholds without air-bone gap.
Graph 2. Type of onset of hearing symptoms.
Graph 3. Presence and type of tinnitus.
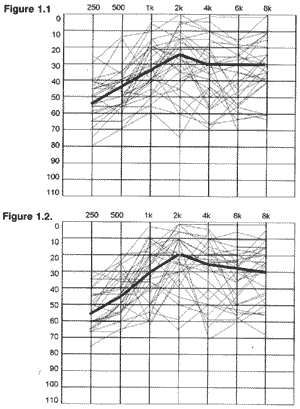
Hearing loss was symmetrical or quite similar in all cases, predominantly mild to moderate (Figures 1.1 and 1.2).
Despite the great variability, the profile of pure tone curves was the following: in six patients there were flat curves, whereas in the other 24 patients there were ascending curves. We did not observe descending curve in any patient.
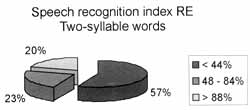
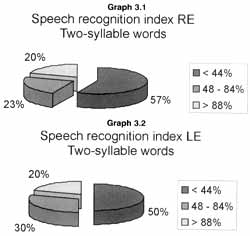
Speech tests were difficult to perform and speech thresholds were not obtained in some cases. The results of speech recognition index with two-syllable words was divided into three categories: up to 44%, from 48 to 84% and better than 88% (Graphs 3.1 and 3.2).
We should bear in mind that many patients came to us with previous hearing tests and some of them had already taken more than two, whose results varied a lot. In some test reports, we found comments made by examiners, such as "poor collaboration", "prone not to answer the questions", or "does not repeat the words".
The results obtained at pure tone audiometry and speech tests depended largely on how the stimuli were presented, usually requiring a longer interval between pure tones and prolonged stimuli. If the test was performed at normal pace, the response was poorer and inconsistent, with many false-positive results. Another peculiarity was that patients presented much more difficulty to recognize low frequency pure tones, whereas middle and high frequency thresholds were easily obtained.
In 1995, we conducted approximately 2,360 audiometric tests in patients over 7 years at the Department of Otorhinolaryngology, Santa Casa de Sao Paulo. We detected sensorineural hearing loss in 869 exams. In the same year, 11 new cases of auditory neuropathy were detected. Therefore, we observed that the occurrence of neuropathy corresponds to 1.26% of the cases of sensorineural hearing loss in our local population. This value should be considered as an estimate, because some patients were submitted to audiometry more than once and we did not report the cases of younger children (who did not take audiometry with earphones). The investigated literature presented very few estimates about the incidence of auditory neuropathy.Chart 3. Occurrence and type of non-auditory neurological abnormality.
Immitance measures
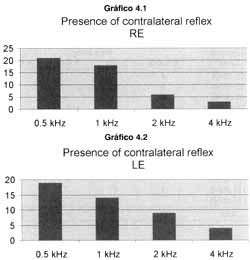
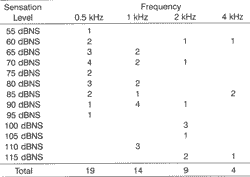
All patients presented A type tympanometric curve. As to presence of contralateral stapedial reflex, the results are presented in Graphs 4.1 and 4.2. Tables 1.1 and 1.2 show the correlation between intensity of stimulus (sensation level in dB) and recording of contralateral reflex.
The presence of the reflex of the stapedial muscle was more frequent in 500 and 1000Hz, opposite to pure tone thresholds that were higher in such frequencies.
The phenomenon of auditory recruitment, a situation in which there is the recovery of hearing sensation as we increase intensity of stimulus in patients with sensorial hearing deficits; is normally identified by the calculation of the difference between the pure tone threshold and the minimal intensity necessary to fire the stapedial reflex. We could notice that in at least 13 situations, the occurrence of the reflex could have had such connotation.
However, we did not find signs and symptoms normally related to recruitment and we noticed that most of the patients did not refer discomfort when we investigated the reflex, even at loud intensities. In addition, many patients did not manage to define the best intensity to perform speech recognition index test, contrasting with the limited comfort range found in recruitment patients.
Figures 1.1 and 1.2. Curves of pure tone audiometry and right and left ear means, respectively.
Graphs 3.1 and 3.2. Results of speech recognition index test with two-syllable words on the right and left ears, respectively.
Brainstem auditory evoked potentials
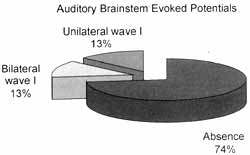
All patients presented abnormalities that varied from partial presence to total absence of potentials (Graph 5).
The study of brainstem auditory evoked potentials used clicks, which are noises located in the frequency range of 1 to 4kHz3. In general, the thresholds we collected were less compromised in this interval of frequencies; however, responses were abnormal in 100% of the patients.
Distortion product otoacoustic emissions
All patients presented responses with amplitudes within the normal parameters for the studied frequencies. The presence of DPOAEs is translated as the presence of outer hair cells with preserved function.
Many authors suggested that the auditory dysfunction had its origin beyond the outer hair cells, in the inner hair cells, the auditory nerve or in the synapses of these elements1,2,7,16,17.
When the difference between hearing threshold and contralateral stapedial reflex is 60dB or lesser, the presence of recruitment is suggested. The comparison of the results of a subjective test (pure tone audiometry) and an objective test (immitance measures) may be critical. The development of assessment techniques for recruitment based on otoacoustic emissions may be the way to follow to understand that issue.
DISCUSSION
Scientific investigations about auditory neuropathy are very comprehensive. Many authors have dedicated to the study of the various sub-issues derived from the topic.
Etiological diagnosis is likely to be genetic if we consider the description of series of cases from the same family, especially in European gypsies2 generally associated with Charcot-Marie-Tooth disease. However, there are other series in which most of the cases have no family history nor additional neurological impairment5,12,17. The onset of auditory neuropathy divides affected people into two main groups: before and after speech development1. Speech acquisition by children with auditory neuropathy is very difficult, not only because of the hearing disorder but also because in this age range etiologies are commonly related to gestational, birth and neonatal complications, such as hyperbilirrubinemia1,4,12. These situations may be followed by cerebral deficits of variable types and severity, and we found a highest incidence of auditory neuropathy and associated neurological deficits in this group. In our ambulatory, we follow up a group of children who have this kind of auditory deficit, who will be described in another study.
Graphs 4.1 and 4.2. Presence of stapedial reflex in different frequencies on the right and left ears, respectively.
Topographical diagnosis of the lesion is investigated and we may find different details and theories. However, authors are unanimous to say that the lesion is in the inner hair cells and the distal portion of the auditory nerve1,2,4,5,7,9,12,16,17. The absence of suppression effect of otoacoustic emissions and stapedial reflex muscle suggested that there is impairment of the peripheral nerve. Hallpike6 described a normal cochlea amidst extremely compromised brainstem and auditory nerve in the autopsy of a patient whose clinical picture suggested auditory neuropathy. Horoupian8 described a similar situation in another patient with family history of progressive neurological compromise associated with hearing loss. Spoendlin15 presented two patients with Friedrich's ataxia and perceptive hearing loss, in which pure tone curve showed elevation of thresholds in low frequencies and pathology of temporal bones revealed preserved cochlea and marked reduction of type I nervous fibers, especially on apical spirals. The author correlated these findings and concluded that hearing loss in low frequencies suggested primary alteration of the auditory nerve.
Berlin1 believes that the term auditory neuropathy should be replaced with auditory dissynchrony, since the transmission of stimulus fail to produce. a uniform depolarization to record the auditorily evoked potentials of the brainstem; however, despite this fact, it reaches the auditory cortex (represented by P300). Other authors have also found long-latency auditory potentials with normal characteristics in subjects with auditory neuropathy10,16,17.
Tables 1.1 and 1.2. Distribution of the presence of stapedial reflex in different frequencies based on sensation level in dB on the right and left ears, respectively.Tables 1.1
Tables 1.2
The scientific community also disagrees unanimously about the use of hearing aids as part of the rehabilitation program of these patients, since the amplification structures are intact (the presence of DPOAEs in normal amplitude suggests the presence of preserved outer hair cells). Some advocate the use of visual aid to develop language in children with auditory neuropathy and, in special cases (poor performance and slow progress), suggest the use of cochlear implant as an attempt to better synchronize auditory stimuli that come from the inner ear to the auditory nerve, describing some (few) good results1. Miyamoto9 compared the result of cochlear implants performed in a child with auditory neuropathy to that of age matches with sensorial alteration. It was noticed that the gain in consonant and words, in open set messages, was far less significant for the child with auditory neuropathy and the author proposed some restrictions to the use of implants in these patients. There are no prospective studies that determine or suggest an optimal rehabilitation strategy. It takes time for studies to be published since there is still a restricted number of cases diagnosed all over the world. The publication of series also provides some conclusions, though less reliable one, depending directly on the size of the sample. There are few large sample studies that enabled description or suggestion of a clinical standard for the patients with auditory neuropathy, which would make diagnosis easier. Starr17 found in a series with 10 auditory neuropathy patients that: 100% of them had onset of symptoms before the age of 30 years; pure tone audiometry in which 50% of the cases had ascending curves and elevation of thresholds, ranging from moderate to severe; compromised speech intelligibility in tested cases; 100% absence of stapedial reflex; presence of P300 in three patients submitted to the test; imaging techniques without alterations correlated with auditory condition, and 30% of the patients with defined neurological deficit (Charcot-Maria-Tooth disease- types I and II).
Graph 5. Results of brainstem auditory evoked potentials.
In our series, we found very similar data and added some further information that may help detection of patients with auditory neuropathy.
We believe that the term auditory neuropathy should be interpreted as a syndromic diagnosis, since it refers to a dysfunction that may have different etiologies, different severity and various levels of compromise, serving as a starting point for specific investigation.
FINAL CONSIDERATIONS
We believe that the results of pure tone audiometry reflected difficulty to identify the tone presented, as described by the patients, but it should not be interpreted as a reduction in the number of outer hair cells and consequent loss of auditory sensitivity. Based on our experience, pure tone curve profile, poor intelligibility and incoherence with the presence of stapedial reflex, either together or separated, in patients whose main complaint is to "not understand people's speech", are evident signs of the presence of auditory neuropathy.
The present report described 30 patients with auditory neuropathy developed after speech acquisition, in which we observed typical auditory complaints and a peculiar display of results of basic audiological battery.
Our suggestion is that, in view of this situation, the patient should be referred to complementary assessment.
REFERENCES
1. BERLIN, C.I. - Auditory Neuropathy. Using OAEs and ABRs from Screening to Management. Seminars in Hearing, 20:304-15, 1999.
2. BUTINAR, D.; ZIDAR, J.; LEONARDIS, L.; POPOVIC, M.; KALYDJIEVA, L.; ANGELICHEVA, D.; SININGER, Y.; KEATS, B.; STARR, A. Hereditary Auditory, Vestibular, Motor, and Sensory Neuropathy in a Slovenian Roma (Gypsy) Kindred. Ann. Neurol., 46.36-44, 1999.
3. CASTRO Jr., N.P.; FIGUEIREDO, M.S. - Audiometria Eletrofisiológica In: LOPES Fo., O.; CAMPOS, C.A.H. -Tratado de Otorrinolaringologia, São Paulo, Roca, 638-50, 1994.
4. DELTENRE, P.; MANSBACH, A.L.; BOZET, C.; CLERCX, A.; HECOX, K.E. - Auditory neuropathy: a report on three cases with early onsets and major neonatal illnesses. EEG and Clin. Neurophys., 104:17-22, 1997.
5. DOYLE, K J.; SWINGER, Y.; STARR, A. - Auditory Neuropathy in Childhood. Laryngoscobe. 108:1374-77. 1998.
6. HALLPIKE, C.S.; HARRIMAN, D.G.F.; WELLS, C.E.C. - A case of afferent neuropathy and deafness. J. Laryng. Otol., 94:945-64, 1980.
7. HOOD, L J. - Auditory Neuropathy: What is it and what can we do about it? Heart, SI: 11-18, 1998.
8. HOROUPLSN, D.S. - Hereditary sensory neuropathy with deafness: A familial multisystem atrophy. Neurology, 39:244-8, 1989.
9. MIYAMOTO, R.T.; KIRK, K.I., RENSHAW, J., HUSSAIN, D. - Cochlear Implantation in Auditory Neuropathy. Laryngoscope, 109:181-5, 1999.
10. MUSIEK, F.E.; BARAN, J.A.; PINHEIRO, M.L. - Neuroaudiology, Case Studies. 11 Ed., San Diego, Singular, 1-277, 1994. .
11. MUSIEK, F.E.; WEIDER, DJ.; MUELLER, R J. - Audiologic Findings in Charcot-Marie-Tooth Disease. Arch. Otolaryngol., 108:595-9, 1982.
12. RHEE, C.; PARK, H.; JANG, Y. - Audiologic Evaluation of Neonates with Severe Hyperbilirrubinemia Using Transiently Evoked Otoacoustic Emissions and Auditory Brainstem Responses. Laryngoscope, 109:2005-8, 1999.
13. ROSENBERG, R.N.; CHUTORIAN, A. - Familial opticoacoustic nerve degeneration and polyneuropathy. Neurology, 17: 827-32, 1967.
14. SPOENDLIN, H. - Optic and Cochleovestibular Degeneration in Hereditary Ataxias. Brain, 97: 41-8, 1974.
15. SININGER, Y.S.; HOOD, LJ.; STARR, A.; BERLIN, C.I.; PICTON, T.W. - Hearing Loss due to Auditory Neuropathy. Audiol. Today, 7.10-3, 1995.
16. STARR, A.; Mc PHERSON, D.; PATTERSON, J.; DON, M.; LUXFORD, W.; SHANNON, R.; SININGER, Y.; TONOKAWA, L.; WARING, M. Absence of both auditory evoked potentials and auditory percepts dependent on timing cues. Brain, 114:1157-80, 1991.
17. STARR, A.; PICTON, T.W.; SININGER, Y.; HOOD, L.3.; BERLIN, C.I. Auditory Neuropathy. Brain, 119: 741-53, 1996.
1 Master degree under study, Faculdade de Ciências Médicas da Santa Casa de São Paulo.
2 Head of the Service of Clinical Audiology, Irmandade da Santa Casa de Misericórdia de São Paulo.
3 Audiologist responsible for the Service of Otoacoustic Emissions, Department of Otorhinolaryngology, Irmandade da Santa Casa de Misericórdia de São Paulo.
4 Second Assistant, Department of Otorhinolaryngology, Irmandade da Santa Casa de Misericórdia de São Paulo. Professor, Discipline of Otorhinolaryngology, Faculdade de Ciências Medicas da Santa Casa de São Paulo.
5 Faculty Professor of Otorhinolaryngology, Faculdade de Ciências Médicas da Santa Casa de São Paulo.
Study conducted at the Department of Otorhinolaryngology, Faculdade de Ciências Médicas and Irmandade da Santa Casa de São Paulo.
Address correspondence to: Osmar Mesquita de Sousa Neto - Rua Professor Artur Ramos, 183, Cjto. 31 - 01454-011 São Paulo/ SP - Tel/Fax: (55 11) 3813-5501.
E-mail: mesquitaneto@uol.com.br
Article submitted on February 20. 2001. Article accepted on March 19. 2001.