Year: 2002 Vol. 68 Ed. 5 - (21º)
Artigo de Revisão
Pages: 736 to 742
Allergic fungal sinusitis: an update
Author(s):
Carla R. Monteiro 1,
Alfeu T. França 2,
Shiro Tomita 3,
Fernando A. Rodrigues 4
Keywords: chronic rhinosinusitis, Aspergillus, allergic fungal sinusitis
Abstract:
Allergic fungal sinusitis is a well recognized form of fungal sinusitis first described 20 years ago. This entity hasn't a well defined diagnostic criteria and many questions rise about its true fisiopathology and treatment. The authors have performed a MEDLINE and LILACS search, comprising the last 20 years, in order to review the most moderns concepts about this new clinical entity. Allergic fungal sinusitis has been considered an important cause of chronic rhinosinusitis and a good relation envolving otolaryngologists, immunologists and pathologists is essential for its correct management.
![]()
INTRODUCTION
Sinusitis is relatively common, affecting approximately 20% of the population1, 2. Fungal sinusitis (FS), however, should be considered in patients with rhinosinusitis (acute or chronic) that failed all antibiotic regimens prescribed to them.
According to Cody et al.3, 10 to 20% of the patients with chronic rhinosinusitis (CR) that are submitted to surgery have a fungus as the main causing agent.
Fungi are the main aeroallergens in many areas. In some areas, for example, spores are hundreds of times more frequent in the inhaled air than in pollen4. The host defense mechanisms against the fungus are not well explained yet, but physiopathogenesis of FS probably involves impaired aeration of the sinus and/or abnormal immune response against the fungus4.
The most widely accepted classification divides FS into acute/fulminating, chronic/indolent, fungal ball and allergic fungal sinusitis (AFS)1, 2, 3.
DeShazo et al.4 reported a fifth type called chronic invasive fungal sinusitis, more prevalent in immunocompromised and diabetes mellitus subjects. This classification is based on presence of tissue invasion, as well as the immune status of the patient. Acute fulminating sinusitis and indolent chronic forms are invasive, but fungus ball and AFS are non-invasive forms, that is, there is no invasion of mucosa or bone by the fungus.
Currently, the most frequently investigated type is allergic fungal sinusitis. The purpose of the present study is to make clinicians, otorhinolaryngologists and allergists aware of the importance of the topic.
LITERATURE REVIEW
HISTORY
In 1971, McCarthy and Pepysapud5,6 observed that 10% of the patients with allergic bronchopulmonary aspergillosis (ABPA) discharged nasal mucous plaques similar to those expectorated from their bronchi during cough. In the study, 41.4% of the 111 patients presented compromise of one of the maxillary sinus shown in simple paranasal sinuses x-rays. The culture of sinusal lavage revealed growth of Aspergillus fumigatus (Af).
Safirstein in 1976apud5,7 described the case of a 24-year-old patient who had ABPA and nasosinusal polyposis (NP). The culture of the affected paranasal sinuses material revealed the growth of Af.
In that same year, Young et al.apud5,8 reported a case of a 15-year-old swimmer who had maxillary and ethmoid sinusitis, NP and left eye proptosis, which led to the expansion of fungal material into the orbit.
In 1981, Millar et al.apud9,10, in Great Britain, published five cases of CR who had histologic characteristics similar to ABPA, whose culture grew Aspergillus sp. They suggested there was a correlation between allergic pulmonary disease and sinusal disease, called paranasal sinuses allergic aspergillosis.
Right after that, in 1983, Katzenstein et al.11, in a retrospective study involving 119 cases of sinusitis, demonstrated that in nine of them there was the indistinguishable histology characteristic of the mucoid impactation in the bronchi of the patients with ABPA. In seven of them, they saw fungal hyphae that were similar to Aspergillus, proposing then the denomination allergic sinusitis by Aspergillus".
Robson et al., in 1989apud3,7,8 introduced the term allergic fungal sinusitis after having identified not only Aspergillus sp, but also other species of fungi that grew in the material removed from the paranasal sinuses.
Ponikau et al.12, in 1999, suggested the substitution of the term AFS by eosinophilic fungal rhinosinusitis, because they believed that there was not enough evidence to confirm that the etiology of this type of FS was caused by a IgE-mediated hypersensitive reaction.
EPIDEMIOLOGY
AFS is considered by some as the most common form of FS4, 13. In addition, it is still the less understood and underdiagnosed affection because it is not familiar to many otorhinolaryngologists, pathologists and radiologists.
Except for the acute/fulminating form, all patients with the other types of FS are immunocompetent. In AFS, there is history of atopy, it normally affects adolescents or young adults, who frequently manifest asthma that is exacerbated by the repetitive episodes of sinusitis9. We can normally find reports of multiple surgeries and high levels of serum eosinophils 14.
Goldstein15 recorded an incidence of 66% of NP among patients with AFS (Figure 1).
Manning et al.13 analyzed 10 patients with AFS. The mean age found was 32 years. Seven were male and three were female patients. Seven of them had history of allergic rhinitis and three, of asthma. All of them had NP and were immunocompetent.
Morpeth et al.10 and Torres et al.8 stated that the real prevalence of AFS is still unknown, being greater in areas where the weather is warm and humid13, 16.
Corey et al.17 believed that the incidence may vary according to geographical variation. In an initial retrospective study by Katzenstein in St. Louis11, an incidence of 4.5% of AFS was confirmed by histopathology. Allphin et al.1, in another retrospective study of patients with CR in Cleveland (Ohio), found an incidence of AFS of 2%, however 6% of the patients with allergic mucin had no hyphae at the histopathology exam or culture (AFS syndrome - like). Cody et al.3, Panda et al.18, Dahniya et al.19, Yagi et al.20 recognized that the incidence of mycosis is high and affects the paranasal sinuses in humid and warm regions, such as the north of India, north of Sudan, Saudi Arabia and southeastern of the United States.
Wickern21 stated that approximately 7% of the patients with CR that require surgery have AFS and most of the fungi are Aspergillus and Bipolaris.
Araújo et al.2, after retrospectively assessing 708 patients with CR submitted to functional endoscopic surgery identified AFS in 11 (1.5%). Out of the total, six presented bronchial asthma and ten NP.
Ponikau et al.12, in a recent study, found one of the greatest indexes of AFS among patients with CR. Of the 101 patients that underwent surgery, 94 (93%) had the diagnosis of AFS, based on histopathology findings and culture results.
ETIOLOGICAL AGENT
Primary etiology agents of AFS that are more commonly discussed in the literature are of the families Moniliaceae and Dematiaceae (pigmented fungi). Dematiaceous differ from Aspergillus for having melanin inside its cells, resulting in a dark color in tissue culture7. They belong to the first family of fungi genus Aspergillus, whose most frequently reported families are Af and A. flavus (Figure 2). In the second family, it is formed by fungi genus Bipolaris, Exserohilum, Curvularia, Alternaria, Cladosporium and Scedosporium17,22. The main species of Bipolaris are: B. spicifera, B. senegalensis, B. hawaiiensis.; Curvularia: C. lunata, C. senegalensis, C. australiensis; Alternaria: A. alternata17.
Moreover, various fungi have already been described, such as Nodulisporium sp16, Fusarium sp16,21, Chrysosporium sp8,16, Rhizomucor8,16, Drechslera16, Exserohilum rostratum22.
Noble et al.16 isolated Epicoccum nigrum in 4 patients with AFS and reported that the most prevalent fungus was Aspergillus sp.
Allphin et al.1 stated that genus Aspergillus is the most frequently found in the environment and isolated in FS. As to AFS, the most prevalent fungi are of the family Dematiaceae10,14,17,22.
However, Schwartz6 reported 10 patients with confirmed diagnosis of AFS and they isolated AF in 6 cases and Curvularia sp in one case.
Whether fungi species associated with AFS have geographical variation is still unknown.
Concomitant infection with more than one fungal species has been rarely reported in the literature5, 22.
PHYSIOPATHOGENESIS
Despite the fact that AFS has been recognized as a distinct clinical entity for over 20 years, many controversies concerning its physiopathogenesis still remain.
Most authors recognize that AFS is more an immune disease than an infectious one7, 9, 17, 23, 24. It is currently advocated that AFS pathophysiology is similar to that of ABPA, owing to the histology similarity between the material found in the paranasal sinuses and that expectorated by the patients with ABPA 10.
AFS is a type I immune response to high total and fungus-specific serum IgE. Moreover, it is possible to have a type III reaction not because of the circulating immunocomplex presence, but because fungus-specific IgG antibodies can be demonstrated. Precipitin and specific serum IgG have also been found3. Serum complement levels are within the normal ranges and there is no cytotoxic activity, as noticed in type II immune reactions17.
Manning et al.24 stated that many factors related to it can lead to progression of AFS. A subject with atopy is exposed to fungal inhalants. The presence of these antigens in susceptible subjects releases an inflammatory response mediated by type I and III reactions. The obstruction of the sinus ostium and the stasis of secretion in the sinus create a favorable environment for fungal growth, increasing further the antigen expression and the immune-mediated inflammatory reaction. A likely by-product of this reaction is allergic mucin, which obstructs the sinus and contributes to perpetuate the pathological process.
The role of type I reactivity in AFS was emphasized in the study24, in which they showed in 9 patients with AFS by Bipolaris high levels of total and Bipolaris-specific IgE.
Mabry and Manning25 analyzed 16 patients with AFS. The sera of these patients were submitted to RAST with fungal antigens and other aeroallergens and were dosed for total serum IgE. Fifteen of the 16 samples showed class II RAST for fungal antigens and other aeroallergens and high levels of IgE in 14 samples.
Cody et al.26 demonstrated a reduction in frequency of ciliary beating in the human nasal respiratory epithelium in vitro when exposed to cultures of Af and Alternaria alternata. This inhibition was maintained even after fungal removal. They concluded that there is one or more substances produced by these fungi that act on the ciliary respiratory epithelium causing inhibition of beating.
The literature is still controversial concerning the role of hypersensitivity versus fungal infection in pathogenesis of AFS. The evidence that speak in favor of the allergic cause is based on the clinical picture manifested by CR in young patients, who were atopic and immunocompetent; presence of allergic mucin and absence of tissue invasion at histopathology; absence of clinical progression of the infection showed by the presence of positive cutaneous test for fungal antigens, such as IgE and IgG specific for these antigens.
Ponikau et al.12, differently from various other authors, stated that there was not enough evidence in the literature to show the participation of IgE-mediated hypersensitivity reactions in the physiopathogenesis of AFS. After the analysis of patients with CR, AFS can be present regardless of atopy. They also stated that the mucosa lesions existing in these patients are resultant from reactions mediated by eosinophils proteins and not IgE.
DIAGNOSIS
Different authors have published their experiences throughout the years in an attempt to define the diagnostic criteria for AFS.
Katzenstein et al.11, in 1983, after having reviewed 119 materials removed from the paranasal sinuses, in which all cultures were positive for Aspergillus sp, stated that allergic mucin was essential for the diagnosis. This material has mucin-like consistency, is thick, green, yellow or brown, similar to peanut butter or cottage cheese. Histologically, there are fungal hyphae, with no spores that invade the mucosa; necrotic cell debris, eosinophil and Charcot-Leyden crystals. These crystals are two-pyramidal hexagonal structures that correspond to the lipophospholysis of the cell membrane of eosinophils and basophils 7. Fungal invasion, formation of granuloma or tissue necrosis are not characteristics present in the histopathology exam of AFS 27. Fungal hyphae in AFS are found dispersed in mucin, many times it is difficult to find them, differently from mycetoma in which they are densely clustered27.
Allphin et al.1, after a retrospective histology analysis of 82 patients with CR identified AFS in 3 cases and the presence of allergic mucin without evidence of fungus in 11 patients. In all cases the following were found: NP, CT scan suggestive of AFS, allergic mucin with non-invasive hyphae, skin and/or serological tests that confirmed atopy.
Bent and Kuhn9, based on the analysis of 15 patients with previous diagnosis of AFS, more emphatically defined some criteria. Five characteristics were identified in all 15 patients, considered therefore, the criteria for the diagnosis. They are: 1) type I hypersensitivity reaction, confirmed by history, skin test or serology; 2) NP; 3) characteristic signs at CT; 4) eosinophilic mucus without tissue fungal invasion (allergic mucin); 5) identification of fungal hyphae in the sinusal material removed during the surgery. Other frequently associated characteristics that were not found in all cases were: asthma, predominance of unilateral lesion, bone erosion, positive culture for fungi, Charcot-Leyden crystals and eosinophilia.
DeShazo et al.5 used 5 well-defined criteria, similar to those described by Bent, but with some specificity. Allergic mucin, which can be identified at rhinoscopy, during the surgery or subsequently to the histopathology tests, presents clinical and histopathology characteristics not found in any other type of FS except for AFS. The fungal elements can be demonstrated through nasal secretion of material collected from the sinus during the surgery (figure 3). They also added that the presence of diabetes should be ruled out, together with other diseases that present immunodeficiency and are treated with immunosuppressant drugs. DeShazo et al. did not include NP, nor skin and serological tests that defined type I hypersensitivity reaction in their criteria because they said that when they are not found, they do not invalidate the diagnosis.
Ponikau et al.12, in a study involving 210 patients with CR, questioned the role of hypersensitivity reactions types I and III in the genesis of AFS. Therefore, they considered only 3 criteria as definite: 1) CR; 2) presence of allergic mucin, and 3) fungal elements of mucin, confirmed by histopathology exam, culture or both.
RADIOLOGY FINDINGS
Some radiographic characteristics, assessed by CT scan and magnetic resonance imaging (MRI) suggest the presence of FS, even though they are not pathognomonic.
At CT scan without contrast, the following signs are present in these patients: focal areas of hyper-attenuation, reactive osteitis, ostiomeatal complex obliteration, bone erosion especially on the nasal lateral wall, and remodeling of the bone walls with expansion to the affected sinusal cavity13. It is important to stress that bone erosion does not mean tissue invasion17. At MRI, at T1 we can find a heterogeneous mass with low intensity signal areas (hypointense) and at T2 these signs can be absent13, 19.
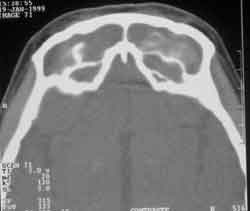
The areas of hyper-attenuation, evidenced by CT scan, are caused by the combination of heavy metals (iron and manganese) and calcium, products of fungal metabolism3, 13 (Figure 4). This characteristics are also seen when there is presence of thick purulent secretion, thrombi, intrasinusal hemorrhage, foreign body and benign and malignant neoplasm, which are all important for differential diagnosis6, 13.
In most of the cases, maxillary and ethmoid sinuses are affected, followed by the sphenoid sinus. Involvement of the frontal sinus seems to be rare. Unilateral affection is more frequent6, 13.
Manning et al.13 analyzed the CT scan and MRI of 10 patients with AFS. In all exams, they identified areas of hyper-attenuation that corresponded surgically to thick allergic mucin. Hypointense signals or their absence, corresponding to the hyper-attenuated areas of the CT scan, were the most common findings at T1 and T2, respectively. All patients presented overflow of sinusal material. Nine patients presented signs of remodeling or bone erosion. The consequent atrophy to intrasinusal pressure and the tissue damage secondary to the eosinophilic degradation can be responsible for the phenomenon described above.
Preoperative diagnosis of AFS, suggested by the CT scan and MRI data, can be extremely valid, since it makes the surgeon and the pathologist consider the need for diagnostic confirmation.
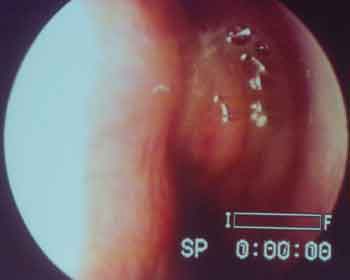
Figure 1. Polyp and allergic mucin filling the left middle meatus of a patient with AFS by Candida sp.
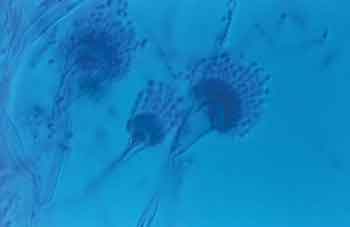
Figure 2. Microscopic aspect of genus Aspergillus sp.
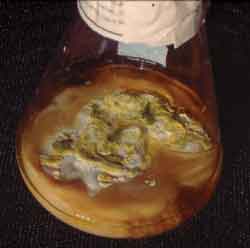
Figure 3. Fungal growth in Sabouraud culture with chloramphenicol of sinusal secretion.
Figure 4. Paranasal sinuses CT scan at axial section, frontal sinus of patient with AFS by Aspergillus sp.
COMPLICATIONS
Despite the fact that AFS follows a slow progression, is not aggressive and does not invade the tissues, the existing intrasinusal pressure can eventually cause expansion and erosion of the sinusal wall, with consequent involvement of the neighboring structures such as the orbit and the brain19.
The most frequently reported sign is proptosis of the ocular globe7, 14. However, diplopia, blepharoptosis, epyphora, ophthalmoplegia and even loss of sight are signs and symptoms also reported and that can be the first manifestation of AFS 14.
Carter et al.14 published six cases of AFS with orbital symptoms. All of them presented proptosis. In two of them there was limitation of ocular mobility. One of them presented symptomatic diplopia and the other was asymptomatic. One patient suffered severe but reversible visual loss.
Marple et al.7, after reviewing 82 cases with AFS, reported visual loss in three cases (3.7%). Orbital involvement without visual loss was found in 14.6% of the patients, and the most commonly found symptoms were proptosis (6.1%) and telecanthus (7.3%). Visual losses were reversible after surgical drainage.
Intracranial complications have also been reported and they are a consequence to bone erosions presented in such cases17, 22.
Kinsella et al.22 reported six cases of AFS that had radiographic evidence of skull base erosion. All patients were submitted to functional endoscopic surgery and in only one case the surgeon had performed dura-mater resection. Except for one patient that maintained abducens nerve paralysis, no other patient presented permanent neurological complication.
TREATMENT
Even though diagnosis has currently been made in a more precise and frequent fashion, the approach of patients with AFS is still a reason for great controversy.
There is no consensus about the ideal treatment, even though most authors combine paranasal sinuses endoscopic functional surgery and local and systemic corticoid.
Kuhn and Bent28 stated: "If fungi are the fact antigens and not infectious agents, the success of the treatment depends on three steps: surgical debridment to remove fungal antigens, allergic mucin and sick polypoid mucosa; to prevent the recurrence of fungal growth, and to modify the immune response of the subject to the antigen."
Since the diagnosis is made, the treatment starts with surgical debridment of nasosinusal material in order to remove the greatest quantity possible of allergic mucin and polypoid mucosa3. This procedure has both diagnostic and therapeutic functions. The surgery provides immediate relief of obstructive symptoms, improving the drainage of secretions and reducing the local fungal load. However, even when using the best surgical technique, it is difficult to completely remove all hyphae and fungal spores.
The medical adjuvant therapy includes systemic corticoids, oral antifungal drugs, immunotherapy or their combination.
The use of nasal and systemic topical corticoids can be started even before the surgery and maintained for a variable postoperative period. Owing to its potent antiinflammatory action and the effects of immunemodulation they are considered the main source of treatment for patients with AFS. Some authors noticed a significant increase in intervals between surgical reviews in patients that received postoperative prolonged corticoid therapy6, 21, 26. Postoperative nasal irrigation with saline solution and endoscopic removal of surgical debris in the ambulatory are also used.
Bent and Kuhn28, 1996, after treating 50 patients with AFS stated that there is no long term cure rate with the treatment currently available. Topical corticoids can be used for local immunemodulation, with no risk of systemic complications, however, it does not cause a great impact on disease control. Systemic corticoids reduce the abnormal immune response and are the main postoperative therapeutic tool. The same authors, after analyzing retrospectively 26 patients with AFS, showed that oral prednisone helped to keep the patient free from the disease for a period of time, but the symptoms resumed as soon as corticoid therapy was finalized.
The use of topical antifungal drugs for the irrigation of nasal fossae after the surgery as an adjuvant therapy was the topic of study for the authors referred above. They analyzed the susceptibility in vitro of 22 fungal cultures of patients with AFS. Ketoconazole and amphotericin B proved to be the most effective. In order to reach maximum benefits, the irrigation should start at the beginning of the surgery and continue for various months in the postoperative period. They believed that irrigation could help fight against the recurrence of the disease by means of blocking fungal growth.
Systemic antifungal drugs are not indicated by most of the authors15, 27, 29 because they do not modify the natural progression of the disease, in addition to increasing the risk of complications inherent to its use.
A even more controversial issue concerns the use of immune therapy with fungal antigens in the control of these patients. In the pathophysiology of AFS there is the participation of type I and III hypersensitivity reactions to fungal antigens. Some authors believe that the likelihood of recurrence seems to be high, if the immune response is not modulated24, 29.
Mabry et al.29,30 have published their experience throughout three years using immunotherapy with fungal antigen and other aeroallergens in patients with AFS and they concluded the following: immune therapy did not exacerbate nasosinusal symptoms; it proved to have its greatest benefit in reducing crusts, avoiding the reaccumulation of allergic mucin; the quick recurrence characteristic of the disease was less frequently observed; the use of nasal and systemic corticoids was less necessary. However, the authors recommended the need for further studies, preferable randomized trials, in order to confirm the beneficial effects of the therapy.
DISCUSSION
AFS is considered the cause of CR, whose non-standardized treatment has not established long-term cure rates of various investigated studies.
The diagnosis of fungal sinusitis initially requires a high level of suspicion by the physician,. since the clinical examination itself is rarely conclusive. In the case of AFS, the suspicion will increase when affecting a young, immunocompetent, atopic subject with chronic sinusitis and NP.
Surgical intervention becomes important both for diagnosis and treatment. Identification of allergic mucin is essential for the characterization of this type of sinusitis and fungus isolation can be made using culture of the collected material and/or histopathology analysis. This is exactly the most crucial point of diagnosis, to confirm the fungal elements of the material collected from the paranasal sinuses.
In the past years, thanks to the more detailed analysis of the nasosinusal mucosa and with improvement in techniques of fungus culture, it became possible to easily identify genus and species more commonly found in each case.
However, some authors still suggest the use of the term allergic fungal sinusitis syndrome-like for the cases of non-identified fungus.
CLOSING REMARKS
There is no doubt that despite the fact that allergic fungal sinusitis has been known for nearly 20 years, many controversial issues concerning physiopathogenesis and consequently, the appropriate treatment, still remain.
Throughout the years, many authors have published their experience leading to different hypotheses, with more questions than responses.
Our suggestion is that more studies be conducted and that physicians should really try to isolate fungi in nasosinusal secretion of patients with chronic rhinosinusitis and NP, in addition to keeping contact with immunologists and pathologists to prevent data from getting lost during the correct diagnosis.
Note: Apud used as reference to authors not listed below, which were quoted by others that are included in the bibliographical references.
REFERENCES
1. Allphin AL, Strauss M, Abdul-Karim FW. Allergic fungal sinusitis: Problems in diagnosis and treatment. Laryngoscope 1991;101:815-820.
2. Araújo E, Palombini BC, Stolz DP, Richter VT. Sinusite fúngica alérgica. F Méd 1999;118:5-11.
3. Cody DT, Neel HB, Ferreiro JA, Roberts GD. Allergic fungal sinusitis: The Mayo Clinic Experience. Laryngoscope 1994;104:1074-1079.
4. DeShazo RD, Chapin K, Swain RE. Fungal sinusitis. N Engl J Med 1997;337:254-259.
5. DeShazo RD, Swain RE. Diagnostic criteria for allergic fungal sinusitis. J Allergy Clin Immunol 1995 jul;96:24-35.
6. Schwartz HJ. Allergic fungal sinusitis: experience in an ambulatory allergy practice. Ann Allergy Asthma Immunol 1996;77:500-502.
7. Marple BF, Mabry RL. Comprehensive management of allergic fungal sinusitis. Am J Rhinol 1998;12:263-8.
8. Torres C, Ro JY, El-Naggar AK, Sim SJ, Weber RS, Ayala AG. Allergic fungal sinusitis: a clinicopathologic study of 16 cases. Hum Pathol 1996;27:793-799.
9. Bent JPIII, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg, 1994;111:580-588.
10. Morpeth JF, Rupp NT, Dolen WK, Bent JP, Kuhn FA. Fungal sinusitis: an update. Ann Allergy Asthma Immunol 1996;76:128-139.
11. Katzenstein AA, Sale SR, Greenberger PA. Allergic Aspergillus sinusitis: a newly recognized form of sinusitis. J Allergy Clin Immunol 1983;72:89-93.
12. Ponikau JU, Sherris DA, Kern EB, Homburger HA, Frigas E, Gaffey TA, Roberts GD. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc 1999;74:877-884.
13. Manning SC, Merkel M, Kriesel K, Vuitch F, Marple B. Computed Tomography and Magnetic Resonance Diagnosis of Allergic Fungal Sinusitis. Laryngoscope 1997;107:170-176.
14. Carter KD, Graham SM, Carpenter KM. Ophthalmic manifestations of allergic fungal sinusitis. Am J Ophthalmol 1999;127:189-95.
15. Goldstein MF, Dunsky EH, Dvorin DJ et al. Allergic fungal sinusitis: a review with four illustrated cases. Am J Rhinol 1994;8:13-18.
16. Noble JA, Crow SA, Ahearn DG, Kuhn FA. Allergic fungal sinusitis in the southeastern USA: involvement of a new agent Epicoccum nigrum Ehrenb. ex Schlecht. 1824. J Medical Veter Micol 1997;35:405-409.
17. Corey JP, Delsupehe KG, Ferguson BJ. Allergic fungal sinusitis: Allergic, infectious, or both? Otolaryngol Head Neck Surg 1995;113:110-119.
18. Panda NK, Sharma SC, ChakrabartiA, Mann SBS. Paranasal sinus mycoses in north India. Mycoses 1998;41:281-6.
19. Dahniya MH, Makkar R, Grexa E, Cherian J, Mattar M. Appearances of paranasal fungal sinusitis on computed tomography. Br J Radiol 1998;71:340-344.
20. Yagi HI, Gumaa SA, Shumo AI, Abdalla N, Gadir AA. Nasosinus aspergillosis in sudanese patients: clinical features, pathology, diagnosis and treatment. J Otolaryngol 1999;28:90-94.
21. Wickern GM. Fusarium allergic fungal sinusitis. J Allergy Clin Immunol 1993;92:624-625.
22. Kinsella JB, Rassekh CH, Bradfield JL, Chaljub G, McNees SW, Gourley WK, Calhoun KH. Allergic fungal sinusitis with cranial base erosion. Head Neck 1996;18:211-217.
23. DeShazo RD. Fungal sinusitis. Am J Med Scienc 1998 jul;316:39-45.
24. Manning SC, Mabry RL, Schaefer SD, Close LG. Evidence of IgE-mediated hypersensitivity in allergic fungal sinusitis. Laryngoscope 1993;103:717-721.
25. Mabry RL, Manning S. Radioallergosorbent microscreen and total immunoglobulin E in allergic fungal sinusitis. Otolaryngol Head Neck Surg 1995;113:721-723.
26. Cody DT, McCaffrey TV, Roberts G, Kern EB. Effects of Aspergillus fumigatus and Alternaria alternata on human ciliated epithelium in vitro. Laryngoscope 1997;107:1511-1514.
27. Schubert MS, Goetz DW. Evaluation and treatment of allergic fungal sinusitis. I. Demographics and diagnosis. J Allergy Clin Immunol 1998;102:387-394.
28. Bent III JP, Kuhn FA. Antifungal activity against allergic fungal sinusitis organisms. Laryngoscope 1996;106:1331-1334.
29. Mabry RL, Manning SC, Mabry CS. Immunotherapy in the treatment of allergic fungal sinusitis. Otolaryngol Head Neck Surg 1997;116:31-35.
30. Mabry RL, Marple BF, Folker RJ, Mabry CS. Immunotherapy for allergic fungal sinusitis: three years' experience. Otolaryngol Head Neck Surg 1998;119:648-651.
1 Master in Otorhinolaryngology, Medical School, Federal University of Rio de Janeiro-UFRJ.
2 Faculty Professor, Allergy and Immunology, Medical School, UFRJ.
3 Head of the Service of Otorhinolaryngology, University Hospital Clementino Fraga Filho (HUCFF), UFRJ.
4 Master studies in Otorhinolaryngology under course, Medical School, UFRJ.
Study conducted at the Services of Otorhinolaryngology and Immunology, UH Clementino Fraga Filho, UFRJ.
Address correspondence to: Carla R. Monteiro - Rua Nilo Peçanha, n° 01/1309 Bl 4, Ingá
24210480 Niteroi - RJ - Tel/Fax: (55 21) 27194320 - e-mail: carlaandreiuolo@zipmail.com.br
Article submitted on September 13, 2001. Article accepted on October 8, 2002