Year: 2013 Vol. 79 Ed. 1 - (13º)
Artigo Original
Pages: 75 to 81
Indications and pitfalls of immunohistochemistry in head and neck cancer
Author(s): Décio de Natale Caly1; Acklei Viana2; Abrão Rapoport3; Rogério Aparecido Dedivitis4; Otávio Alberto Curioni5; Cláudio Roberto Cernea6; Lenine Garcia Brandão7
DOI: 10.5935/1808-8694.20130013
Keywords: carcinoma, squamous cell; head and neck neoplasms; immunohistochemistry; lymphoma; sarcoma.
Abstract:
Immunohistochemistry (IHC) has been employed in the differential diagnosis of tumors.
OBJECTIVE: To assess the use of IHC in cases of head and neck tumor.
METHOD: This is a retrospective study of the cases included in the Cancer Registry of the institution.
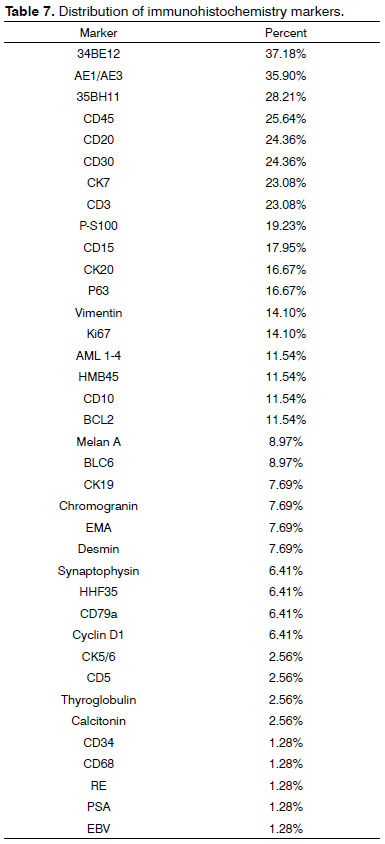
RESULTS: IHC was used in 76 (11%) of 704 pathology tests. Most cases were carcinomas (85.80%), and 83.66% of them were squamous cell carcinomas. All tests were done with diagnostic purposes. The most frequently used antibodies were 34BE12 (37.18%), AE1/AE3 (35.9%), 35BH11 (28.21%), CD45 (25.64%), CD20 (24.36%), CD30 (24.36%), CK7 (23.08%) and CD3 (23.08%).
CONCLUSIONS: IHC was used in 10.67% of the head and neck tumor cases submitted to pathology testing, mostly for carcinoma (5.26%). In the determination of squamous cell carcinoma, IHC accounted for 18.42% of all tumors.
![]()
INTRODUCTION
Macro and microscopic alterations lie in the basis of pathology diagnostics, and microscopic morphology plays a fundamental role in the interpretation of histogenesis, classification, and prognostic assessment of tumors. However, in the 1970s more elaborate techniques gained significant ground in aiding in the diagnosis of cases in which the assessment produced from the use of the usual histochemical stains was not conclusive. Immunohistochemistry (IHC) protocols were developed in this setting, using antibodies tagged with chromogens to identify specific markers. In these protocols, antigen-antibody reactions using non-fluorescent chromogens are analyzed in an optical microscope. IHC is performed in paraffin-embedded specimens sliced on a microtome and submitted to treatments for antigen retrieval, processed with immunoperoxidase and development reagents1,2. The ease of handling paraffin-embedded specimens has granted significant popularity to IHC in pathology labs.
Despite the relevant contributions offered by IHC, the assessment of the specimens and the determination of which panel to use is made only after careful analysis by the pathologist through traditional pathology testing protocols.
The main indications for IHC are: definition of histogenesis; differential diagnosis between reactive and neoplastic states; etiologic diagnosis of infectious diseases; determination of prognostic factors; determination of target therapy sites; location of primary tumors in malignant disease; determination of specific products (such as hormones and proteins); and cancer subtyping.
In the definition of histogenesis, assessment is carried out to determine the tissue and/or cell origin of a specific cell population. In the case of neoplasms, IHC provides substantial help in defining whether the tumor derives from epithelial, mesenchymal, hematopoietic, or nervous tissue.
The criteria to define whether a tumor is benign or malignant are still strongly founded in morphology. Macroscopically, features such as infiltrative growth pattern, borders of the tumor, absence of a tumor capsule, growth rate, necrosis, and hemorrhage are considered. Microscopically, cell count and anaplasia (cell and nucleus pleomorphism, atypical mitosis, and nuclear-cytoplasmic ratio) are analyzed.
It is important that these ideas be considered, as when IHC is used to determine histogenesis, the tests yield positive results for the same markers based on tissue origin and not on whether the tumor is benign or malignant, i.e., normal malpighian (squamous) epithelium will be marked the same way as squamous cell carcinoma.
Histological classification is based on the criteria defined by Broders, in which cell differentiation and keratinization of tumor cells are categorized into four grades of final differentiation3. Squamous cell carcinoma (SCC) may present the following variants, according to the classification of the World Health Organization: verrucous, basaloid, papillary, spindle-cell, acantholytic, adenosquamous, and cuniculatum4.
However, neoplasms other than SCC may involve the head and neck area, including undifferentiated carcinomas, metastasis, neuroendocrine carcinomas, sarcomas, lymphomas, etc.
In this setting IHC is required to produce diagnosis and subtype tumors. Despite the routine use of IHC and its ample availability for clinical and surgical personnel, it is worthwhile discussing its use and precise indications in diagnostics, prognostics, the possible impacts it may have upon therapy, and the technique's limitations. Thus, this paper aimed to assess the use of IHC in cases of head and neck cancer.
METHOD
This retrospective study looked into the pathology test results of the Cancer Registry of the institution dated from April 2009 to February 2012, and included cases of the hospital's Head and Neck Service.
Enrollment criteria included: subjects diagnosed and treated at the institution with cases entered into the Hospital Cancer Registry; pathology tests filed in electronic format in the Hospital Cancer Registry. Exclusion criteria: lack of IHC testing support; thyroid tumors; basal-cell carcinoma of the skin and cutaneous attachments; and odontogenic tumors.
The study included cases processed by IHC protocols, considering the incidence rates of various tumor types - carcinomas, sarcomas, and lymphomas - and utilized panels.
Descriptive analysis was performed based on absolute and relative frequencies.
RESULTS
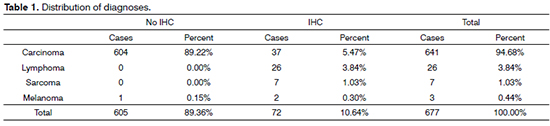
A grand total of 677 pathology test results were selected; IHC was not performed in 605 (89.4%) cases; seventy-two (10.6%) cases had IHC test results.
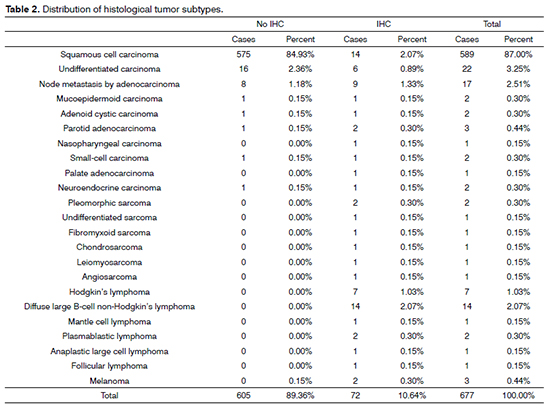
Considering the split of tumor types, carcinomas accounted for 94.69% of the cases, followed by lymphomas (3.84%). IHC panels for diagnostic purposes were used in 10.64% of the cases, 5.47% of which were epithelial tumors. IHC was done in all cases of lymphoma and sarcoma (Table 1). In terms of histological subtypes, SCC accounted for most cases (87%). The other subtypes are listed on Table 2.
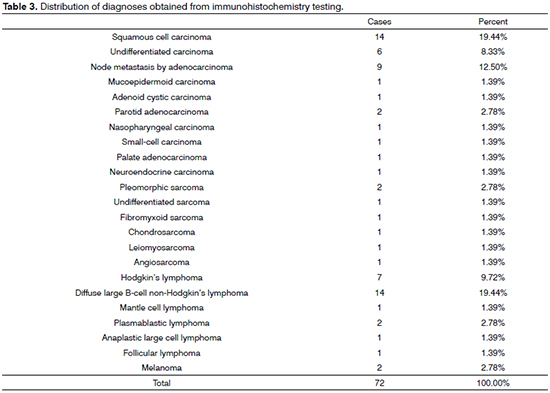
In the 72 cases submitted to immunohistochemistry testing, SCC (19.44%) and diffuse large cell lymphomas tied for first (19,44%), followed by node metastasis (12.50%), Hodgkin's lymphoma (9.72%), and undifferentiated carcinoma (8.33%) (Table 3).
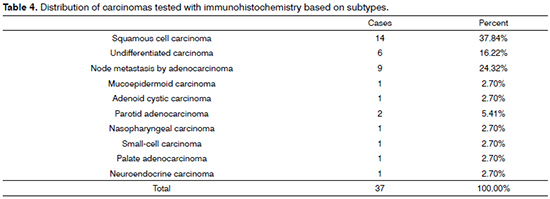
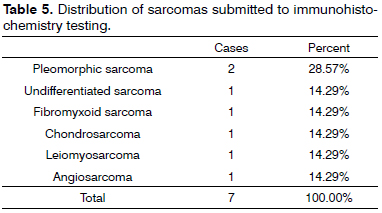
When incidence rates within specific tumor groups (carcinoma, lymphoma, sarcoma) were compared for diagnosed subtypes for which IHC was done, the highest rates in the carcinoma group went to SCC, with 37.84%; in the lymphoma group, diffuse large B-cell lymphoma ranked first, with 53.85%; and in the sarcoma group, pleomorphic sarcoma was the most commonly observed tumor type, with 28.57% (Tables 4, 5, and 6).
The 76 immunohistochemistry tests were done with diagnostic purposes. Considering the chosen IHC panels and antibodies, 34BE12 (37.18%), AE1/AE3 (35.90%), 35BH11 (28.21%), CD45 (25.64%), CD20 (24.36%), CD30 (24.36%), CK7 (23.08%) and CD3 (23.08%) were the most frequently used (Table 7).
DISCUSSION
Immunohistochemistry has significantly improved the outlook for pathology tests. The possibility of obtaining clarification on once doubtful and hard-to-diagnose tumors turned this technique into a powerful addition to the roster of routine test protocols.
Epithelial tissues can be immunolabeled by cytokeratins, proteins present in the cytoskeleton of epithelial cells. There are more than 25 types of cytokeratin divided into low and high molecular weight subtypes. Specific tissue types are labeled by specific cytokeratins. Squamous epithelium is labeled by high molecular weight cytokeratins such as CK4, CK5, CK6, CK13, CK14, CK17 or by a cocktail of these cytokeratins called 34βE12. Glandular epithelium is preferentially labeled by low molecular weight cytokeratins (CK8, CK18, CK 19) or by a low molecular weight cocktail called 35β11.
In diagnostic terms, IHC in SCC has limited effectiveness in subtypes that escape more traditional forms of disease. Poorly differentiated carcinomas in which histogenesis is needed for the differential diagnosis against other tumors such as sarcoma, undifferentiated, small-cell and neuroendocrine tumors, also challenge the performance of IHC protocols. SCC is labeled by the pan-cytokeratin stain (AE1/AE3) and high molecular weight cytokeratin complex 34βE12; keratinizing SCC of the nasal cavity and paranasal sinuses, basaloid SCC, and spindle-cell SCC may require additional markers. Even when looking into or confirming the diagnosis of SCC, it is important to resort to other markers to perform proper differential diagnosis against other tumor types (mesenchymal, hematopoietic, adenocarcinomas).
Even though IHC has limited indication for head and neck SCC, recent studies have assessed its prognostic relevance. Molecular pathways are known to participate in carcinogenesis. Among the most studied are the p53, p16, cyclin D1, and PTEN pathways. The association between p53, cyclin, and p16 labeling and tumor thickness, metastasis, and response to adjuvant therapy has been analyzed. Correlations have been drawn, but no consensus has been reached for head and neck SCC5-7. The predictive value of therapeutic response to radiotherapy has been targeted by some authors, in an attempt to correlate the degree of response to the degree of PTEN expression by IHC8,9.
Significant attention has been devoted to the use of target therapies in managing tumors. The epidermal growth factor receptor (EGFR) pathway acts as an oncogene by activating cell proliferation and division. When this pathway suffers mutations or is activated, keratinocytes are stimulated to proliferate and grow. The EGFR pathway is mutated in about 30% of the cases of head and neck SCC. EGFR expression has been correlated to poorer prognosis10. The use of monoclonal antibodies to prevent EGFR activation has garnered significant interest. The detection of this receptor through IHC aids in the choice of therapy and use of specific medication11.
Tumor growth and propagation are directly linked to the degree of angiogenesis. Vascular endothelium growth factor (VEGF), described in the 1980s, is a glycoprotein that binds to specific receptors to act on the mechanism of angiogenesis. Angiogenesis is essential for tumor growth and propagation and correlates with poorer prognosis. More recently, IHC has been used in the VEGF pathway to assess patient prognosis; drugs such as bevacizumab have been used to block the VEGF pathway and attain better control over the disease12,13.
IHC has played a significant role in primary tumor site identification in metastatic disease. Panels labeling SCC, adenocarcinoma, thyroid carcinoma, sarcoma, lymphoma, neuroendocrine carcinoma, and melanoma can improve diagnostic accuracy and thus provide valuable input to the determination of the most effective course of therapy.
All in all, IHC is a good ancillary technique. In order to optimize its use, clear indication criteria are needed. The technique helps approach head and neck tumors, mainly in diagnosing sarcomas and lymphomas and enabling their adequate classification. Its use is limited in cases of carcinomas characterized by diagnostic doubt.
In our series, most tumors were categorized as SCC (87%), as also reported in the literature14,15, and did not pose significant diagnostic difficulties. IHC was used in only 10.67% of the cases of carcinoma, when further clarification was needed for diagnosis and histogenesis. The use of IHC to confirm histogenesis and tumor subtype is supported by the literature16,17. The number of cases in our series in which IHC protocols were applied to identify carcinomas (5.77%) suggests that immunohistochemistry has not been widely used in our practice or yielded significant impact upon changes in therapy.
IHC is a useful method to identify primary tumor sites in patients with node metastasis18. In our series, IHC was needed in 11.69% of the cases. Although this is not a significant number, the relevance of IHC in indicating the correct histogenesis of the neoplasm in cases of unidentified primary tumor and consequently enhancing the effectiveness of adjuvant therapy cannot be neglected.
IHC has undisputed value for other tumor types (sarcomas and lymphomas). It has been used in the determination of histogenesis and tumor subtyping, and has consequently had significant impact on the choice of therapy.
CONCLUSION
Immunohistochemistry was used in 10.67% of the head and neck tumor cases tested with traditional pathology protocols. IHC was more frequently used in carcinomas, accounting for 5.26% of all cases. In the determination of squamous cell carcinoma, IHC protocols were used in 18.42% of the tumors. The diagnosis of squamous cell carcinoma is still done largely without the use of immunohistochemistry panels. IHC is required to confirm the diagnosis in cases of metastatic disease. It is also needed in the diagnosis and subtyping of lymphomas and subtyping of sarcomas.
REFERENCES
1. Heyderman E. Immunoperoxidase technique in histopathology: applications, methods, and controls. J Clin Pathol. 1979;32(10):971-8.
2. Heyderman E, Neville AM. A shorter immunoperoxidase technique for the demonstration of carcinoembryonic antigen and other cell products. J Clin Pathol. 1977;30(2):138-40.
3. Broders AC. Squamous-cell epithelioma of the lip: a study of five hundred and thirty-seven cases. JAMA. 1920;74(10):656-64.
4. Barnes L, Everson JW, Reichart P, Sidransky D, editors. World Health Organization Classification of tumours. Pathology and genetics of head and neck cancer. Lyon: IARC Press; 2005.
5. Peltonen JK, Vähäkangas KH, Helppi HM, Bloigu R, Pääkkö P, Turpeenniemi-Hujanen T. Specific TP53 mutations predict aggressive phenotype in head and neck squamous cell carcinoma: a retrospective archival study. Head Neck Oncol. 2011;3:20.
6. Fischer CA, Kampmann M, Zlobec I, Green E, Tornillo L, Lugli A, et al. p16 expression in oropharyngeal cancer: its impact on staging and prognosis compared with the conventional clinical staging parameters. Ann Oncol. 2010;21(10):1961-6.
7. Feng Z, Guo W, Zhang C, Xu Q, Zhang P, Sun J, et al. CCND1 as a predictive biomarker of neoadjuvant chemotherapy in patients with locally advanced head and neck squamous cell carcinoma. PLoS One. 2011;6(10):e26399.
8. Lee JI, Soria JC, Hassan KA, El-Naggar AK, Tang X, Liu DD, et al. Loss of PTEN expression as a prognostic marker for tongue cancer. Arch Otolaryngol Head Neck Surg. 2001;127(12):1441-5.
9. Snietura M, Jaworska M, Mlynarczyk-Liszka J, Goraj-Zajac A, Piglowski W, Lange D, et al. PTEN as a prognostic and predictive marker in postoperative radiotherapy for squamous cell cancer of the head and neck. PLoS One. 2012;7(3):e33396.
10. Elferink LA, Resto VA. Receptor-tyrosine-kinase-targeted therapies for head and neck cancer. J Signal Transduct. 2011;2011:982879.
11. Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol. 2010;7(9):493-507.
12. Tanaka T, Ishiguro H, Kuwabara Y, Kimura M, Mitsui A, Katada T, et al. Vascular endothelial growth factor C (VEGF-C) in esophageal cancer correlates with lymph node metastasis and poor patient prognosis. J Exp Clin Cancer Res. 2010;29:83.
13. Argiris A, Karamouzis MV, Gooding WE, Branstetter BF, Zhong S, Raez LE, et al. Phase II trial of pemetrexed and bevacizumab in patients with recurrent or metastatic head and neck cancer. J Clin Oncol. 2011;29(9):1140-5.
14. Stelow EB, Mills SE. Squamous cell carcinoma variants of the upper aerodigestive tract. Am J Clin Pathol. 2005;124 Suppl:S96-109.
15. Wenig BM. Squamous cell carcinoma of the upper aerodigestive tract: precursors and problematic variants. Mod Pathol. 2002;15(3):229-54.
16. Emanuel P, Wang B, Wu M, Burstein DE. p63 Immunohistochemistry in the distinction of adenoid cystic carcinoma from basaloid squamous cell carcinoma. Mod Pathol. 2005;18(5):645-50.
17. Tsubochi H, Suzuki T, Suzuki S, Ohashi Y, Ishibashi S, Moriya T, et al. Immunohistochemical study of basaloid squamous cell carcinoma, adenoid cystic and mucoepidermoid carcinoma in the upper aerodigestive tract. Anticancer Res. 2000;20(2B):1205-11.
18. Park JM, Jung CK, Choi YJ, Lee KY, Kang JH, Kim MS, et al. The use of an immunohistochemical diagnostic panel to determine the primary site of cervical lymph node metastases of occult squamous cell carcinoma. Hum Pathol. 2010;41(3):431-7.
1. PhD in Pathology from A.C. Camargo Hospital, São Paulo (Pathologist at the Heliópolis Hospital, São Paulo).
2. Resident Physician in the Department of Head and Neck Surgery and Otorhinolaryngology at the Heliópolis Hospital, São Paulo.
3. Associate Professor in the Department of Surgery at the Medical School of the University of São Paulo (Technical Director in the Department of Health at the Heliópolis Hospital, São Paulo).
4. Associate Professor. Supervisor of the Larynx Group in the Department of Head and Neck Surgery of the University Hospital of the Medical School of the University of São Paulo (MD).
5. PhD in Pathology from the Medical School of the University of São Paulo (Head of the Department of Head and Neck Surgery and Otorhinolaryngology at the Heliópolis Hospital, São Paulo).
6. Associate Professor in the Department of Head and Neck Surgery at the Medical School of the University of São Paulo, São Paulo.
7. Professor in the Department of Head and Neck Surgery at the Medical School of the University of São Paulo, São Paulo.
Department of Head and Neck Surgery and Otorhinolaryngology at the Heliópolis Hospital, São Paulo and Department of Head and Neck Surgery at the Medical School of the University of São Paulo, São Paulo.
Send correspondence to:
Rogério Aparecido Dedivitis
Rua Cônego Xavier, nº 276. Sacomã
São Paulo - SP. Brazil. CEP: 04231-030
E-mail: dedivitis@usp.br
Paper submitted to the BJORL-SGP (Publishing Management System - Brazilian Journal of Otorhinolaryngology) on July 26, 2012.
Accepted on October 15, 2012. cod. 9522.