Year: 2012 Vol. 78 Ed. 5 - (4º)
Artigo Original
Pages: 22 to 26
Comparing the clinical profile of non obese children with sleep apnea and snoring
Author(s): Daniele de Oliveira Soares Stefanini1; Emília Leite de Barros2; Renato Stefanini3; Márcia Lurdes de Cássia Pradella-Hallinan4; Shirley Shizue Nagata Pignatari5; Reginaldo Raimundo Fujita6
DOI: 10.5935/1808-8694.20120004
Keywords: child, metabolic diseases, polysomnography, sleep apnea, obstructive.
Abstract:
Few studies in the literature have looked into the cardiovascular and metabolic effects of Obstructive Sleep Apnea Syndrome (OSAS) in children.
OBJECTIVE: This study aims to evaluate the metabolic profile of non-obese children with OSAS.
METHODS: Fifty-two children were enrolled in this study, 21 girls and 31 boys. Patients were divided into two groups: OSAS (28 children) and Snore (22 children) according to polysomnographic evaluation. All children were submitted to ENT examination, measurements of weight, height and blood pressure. Blood samples were tested for hemoglobin, hematocrit, fasting glucose, fasting insulin, triglycerides, total cholesterol, HDL, LDL, VLDL, TSH and T4. The gathered data sets were compared between groups and also within the OSAS group according to the severity of the syndrome.
RESULTS: The children from both groups had no alterations in blood pressure levels. The results of the blood tests were normal for both groups. Results of hemoglobin, hematocrit, and HDL were all significantly higher in the Snore group when compared to the OSAS group; by their turn, VLDL levels were higher in the OSAS group. There was no statistical difference between the groups based on OSAS severity.
CONCLUSION: Non-obese children with OSAS present no significant alterations in metabolic tests or blood pressure levels.
![]()
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is a respiratory sleep disorder that affects adults and children. It is characterized by repeated episodes of upper airway obstruction accompanied by intermittent hypoxia and hypercapnia1.
Prevalence rates reported in the literature range between 0.7% and 3%. Incidence rates peak among preschoolers, as this age group is more affected by adenotonsillar hypertrophy, the main cause of OSAS in children1. The treatment of pediatric OSAS is adenotonsillectomy or, in rare cases, CPAP1-3.
OSAS may have severe clinical consequences such as cor pulmonale, in addition to other cardiovascular and metabolic disorders, delays in weight and height gain, facial and chest skeletal alterations, nocturnal enuresis, behavior and learning disorders, and cognitive impairments1,4.
The association between OSAS and systemic high blood pressure and other cardiovascular and metabolic diseases has been well documented1,5-8. Sleep disorders and intermittent nocturnal hypoxemia in combination with OSAS have been related to metabolic disorders including altered glucose metabolism and dyslipidemia. Evidence suggests an association between OSAS and diabetes mellitus type 2, glucose intolerance, and insulin resistance9.
Studies in pediatric populations have shown an association between OSAS and alterations in systemic blood pressure levels10,11, echocardiographic findings12,13 and insulin resistance14,15. These alterations seem to be present mainly in children with moderate to severe OSAS and in obese kids16. Only a few studies have looked into the consequences of OSAS in non-obese children.
This study compared the clinical profiles of non-obese children with OSAS and primary snoring.
METHOD
The study was approved by the Research Ethics Committee of the institution and granted permit CEP 1814/08. Pediatric patients seen in an outpatient setting between March of 2009 and August of 2010 were enrolled. The subjects were aged between three and 13 years and were predominantly mouth breathers. They had been snoring three or more days per week for at least six months and had a body mass index (BMI) equal to or under the 95th percentile based on age and gender. Obese and diabetic subjects and individuals with cardiovascular, metabolic or neuromuscular diseases, genetic syndromes or craniofacial malformations were excluded.
The patients underwent complete ENT examination and had their weight, height, and blood pressure (BP) measured . BP was measured with the patients seated after resting for at least 20 minutes. A proper cuff for the patients' age and weight was used. BP measurements were categorized as 'altered' or 'unaltered' in accordance with the second release of the table published by the National Task Force on Hypertension of the National Heart, Lung and Blood Institute endorsed by the American Academy of Pediatrics in 1987 and reviewed in 199617,18. Systolic or diastolic BP values under the 90th percentile were considered normal.
Tonsillar hypertrophy was categorized in four degrees as per the scheme proposed by Brodsky19, while pharyngeal tonsil hypertrophy was assessed through nasopharyngeal endoscopic examination. Grades III and IV were considered as obstructive palatine tonsil hypertrophy. Obstruction caused by the pharyngeal tonsil was characterized when it occupied 70% or more of the cavum area.
All subjects underwent polysomnography. Obstructive apnea was characterized as the presence of inspiratory effort in the absence of airflow for at least two respiratory events. Hypopnea was defined as a reduction of at least 50% in airflow amplitude in the presence of inspiratory effort and oxyhemoglobin desaturation of at least 4%, or the subject awakening. The apnea hypopnea index (AHI) was defined as the set of obstructive and mixed (central and obstructive components) apnea and hypopnea episodes per hour of sleep20. OSAS was defined as the presence of AHI > 1. If AHI > 1 and < 5, the patient had mild OSAS; if AHI > 5 and < 10, the subject had moderate OSAS; severe cases had AHI > 10.
Fasting blood samples were taken to assess hemoglobin (Hb), hematocrit (Ht), fasting glucose, fasting insulin, triglycerides, total cholesterol and cholesterol fractions (HDL, LDL, VLDL), TSH and free T4 levels.
Non-parametric tests were used in this study.
The test for equality of two proportions was used to compare whether the proportions of responses of two variables and/or their levels are statistically significant. The Mann-Whitney test was used to compare variables from independent samples two by two. The Kruskal-Wallis test was used to compare the three degrees of OSAS severity to all quantitative variables.
Spearman's rank correlation ratio was used to measure the correlation between all variables and the OSAS degree of severity. The correlation test was used to validate the correlations.
Significance was set at 0.05 (5%). All confidence intervals used in this study had a 95% statistical confidence level.
RESULTS
Fifty-two children were enrolled in the study, 21 (40.38%) of them being girls and 31 (59.61%) boys. The patients were divided into two groups: OSAS (AHI > 1 event per hour) and Snoring (AHI < 1 event per hour). Both groups were homogeneous in terms of gender and age. Twenty-eight children were included in the Snoring Group (13 females and 15 males), while the OSAS Group had 24 subjects (8 females and 16 males). In the OSAS Group, eleven children had mild OSAS, seven had moderate OSAS, and six had severe OSAS. Mean ages were 8.07 years in the Snoring Group and 6.67 years in the OSAS Group.
All subjects had obstructive OSAS caused by hypertrophied lymphoid tissue. In the OSAS Group, 18 (75%) children had hypertrophied pharyngeal tonsils and 15 (62.5%) had hypertrophied tonsils. In the Snoring Group, 13 (46.4%) individuals had hypertrophied pharyngeal tonsils and 10 (35.7%) had hypertrophied tonsils.
The subjects from both groups had unaltered BP levels.
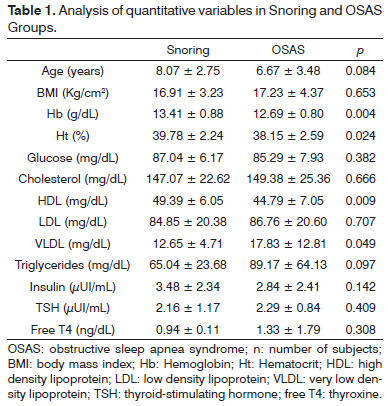
The groups were compared for quantitative variables age, BMI, Hb, Ht, fasting glucose, total cholesterol, cholesterol fractions, triglycerides, insulin, TSH, an free T4 as described on Table 1.
The studied variables had normal values in both groups. However, statistically significant differences were found between the groups for Hb, Ht, HDL, and VLDL. Among these, only VLDL was higher in the OSAS Group than in the Snoring Group.
The subjects in the OSAS Group were analyzed to compare the three degrees of syndrome severity and their quantitative variables. The differences observed between the subjects with various degrees of OSAS severity were not statistically significant.
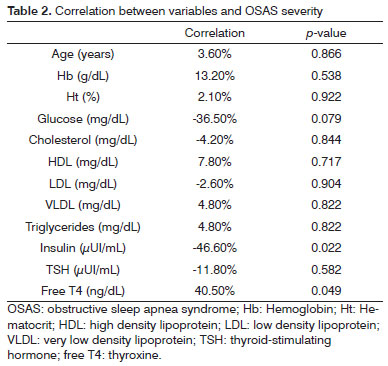
The possible correlations between all variables and OSAS severity were analyzed for the OSAS Group (Table 2).
There was a negative correlation between the degree of severity and insulin (-46.6%) and a positive one with free T4 (40,5%). Both correlations were rated as Fair.
DISCUSSION
Many papers in the literature have shown that children with OSAS have altered BP levels10,21,22. Altered BP levels were not observed in this study for the non-obese children of either of the groups. Kaditis et al.16 also failed to show altered BP levels in non-obese children with OSAS, but we found significantly higher BP values in the OSAS Group when compared to subjects in the control group. The study done by Amin et al.12 in 2002 did not reveal altered BP levels in children with OSAS or in primary snorers.
The metabolic indices of the patients in both groups were not altered. Only VLDL was increased and HDL cholesterol was significantly lower in children with OSAS. Hemoglobin and hematocrit levels were significantly higher in the patients without OSAS. Kaditis et al.16 studied non-obese children with OSAS and did not find differences in total cholesterol, cholesterol fractions, triglycerides, and insulin levels when compared to children without OSAS.
Only patients with OSAS were checked to see whether there was any variation in their metabolic indices in association with OSAS severity, but no statistically significant differences were found between OSAS severity and the analyzed metabolic indices. It was observed that increasing degrees of disease severity led to lower insulin levels and higher free T4 values.
These results vary from the ones reported by de la Eva et al.14, in which a positive correlation was established between OSAS severity and insulin levels in children regardless of BMI. In China, obese children with OSAS were studied, and increased levels of insulin were found in this group, in a positive correlation with OSAS severity23. Redline et al.10 studied a group of teens and showed that adolescents with sleep respiratory disorders have seven times more chance of having metabolic alterations than adolescents with no sleep disorders. After adjustments for gender and BMI, these adolescents had higher levels of BP, insulin, and LDL cholesterol.
Nonetheless, the study conducted by Tauman et al.24 verified that obesity, and not OSAS, is the determining factor for insulin resistance and metabolic alterations seen in their cohorts. This study can explain why our study and in the one by Kaditis no metabolic alterations were found in non-obese children with and without OSAS. It appears that fat tissue plays an important role as a mediator for cytokines, growth factors, and sex steroids, which alter the action and secretion of insulin25.
A longitudinal study done with children showed that metabolic alterations are more evident between 10 and 19 years of age26. The differing results seen in the studies mentioned above may be related to the variations in age of the studied populations. Positive findings may prevail in older children and in adolescents, as they have been enduring the consequences of OSAS for longer than younger subjects, not to mention the impact of puberal changes.
Another confounding factor in OSAS pediatric studies is obesity, which in some papers is reported as a determining factor for the presence of metabolic alterations while not in others. More studies are required to look into children with OSAS and metabolic alterations by separating obese from non-obese subjects and analyzing them by age range.
Our study looked only at non-obese children to isolate the consequences associated with OSAS from obesity. Age is known to affect the onset of metabolic alterations. Therefore, it is important to divide subjects based on their ages. However, given the difficulties inherent to polysomnographic examination, our sample had a limited number of subjects and could not be further divided into age-based subgroups.
The literature on adult OSAS is extensive, but few studies have been carried out on pediatric sleep respiratory disorders. The papers on children with OSAS and metabolic and inflammatory disorders have diverging results, mainly because of the myriad of methods employed to categorize OSAS and the variations in the data related to subject age. Adolescents appear to have different metabolic characteristics than younger children, but they have been studied together in most papers, which have thus failed to recognize the age-related variations.
CONCLUSION
Non-obese children with OSAS did not present significant blood pressure or metabolic alterations when compared to pediatric primary snoring patients.
REFERENCES
1. Weber SAT. Avaliação cardíaca em crianças com distúrbios respiratórios obstrutivos, antes e após adenotonsilectomia [Tese de Doutorado] Botucatu: Faculdade de Medicina da Universidade Estadual Paulista; 2006.
2. Görür K, Döven O, Unal M, Akkus N, Ozcan C. Preoperative and postoperative cardiac and clinical findings of patients with adenotonsillar hypertrophy. Int J Pediatr Otorhinolaringol. 2001;59(1):41-6.
3. Pereira Jr JC, Pessoa JHL. Síndrome da apnéia/hipopnéia do sono: uma visão pediátrica. Rev Paul Pediatria. 2005;23(4):184-91.
4. Pac A, Karadg A, Kurtaran H, Aktas D. Comparison of cardiac function and valvular damage in children with and without adenotonsillar hypertrophy. Int J Pediatr Otorhinolaringol. 2005;69(4):527-32.
5. Quan SF, Gersh BJ; National Center on Sleep Disorders Research; National Heart, Lung, and Blood Institute. Cardiovascular consequences of sleep-disordered breathing: past, present and future: report of a Workshop from the National Center on Sleep Disorders Research and the National Heart, Lung, and Blood Institute. Circulation. 2004;109(8):951-7.
6. Caples SM, Garcia-Touchard A, Somers VK. Sleep-disordered breathing and cardiovascular risk. Sleep. 2007;30(3):291-303.
7. Kasasbeh E, Chi DS, Krishnaswamy G. Inflammatory aspects of sleep apnea and their cardiovascular consequences. South Med J. 2006;99(1):58-67.
8. Tsaoussoglou M, Bixler EO, Calhoun S, Chrousos GP, Sauder K, Vgontzas AN. Sleep-disordered breathing in obese children is associated with prevalent excessive daytime sleepiness, inflammation, and metabolic abnormalities. J Clin Endocrinol Metab. 2010;95(1):143-50.
9. Seicean S, Kirchner HL, Gottlieb DJ, Pinjabi NM, Resnick H, Sanders M, et al. Sleep disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: the Sleep Heart Health Study. Diabetes Care. 2008;31(5):1001-6.
10. Redline S, Storfer-Isser A, Rosen CL, Johnson NL, Kirchner HL, Emancipator J, et al. Association between metabolic syndrome and sleep-disordered breathing in adolescents. Am J Respir Crit Care Med. 2007;176(4):401-8.
11. Amin RS, Carroll JL, Jeffries JL, Grone C, Bean JA, Chini B, et al. Twenty-four-hour ambulatory blood pressure in children with sleep-disordered breathing. Am J Resp Crit Care Med. 2004;169(8):950-6.
12. Amin RS, Kimball TR, Bean JA, Jeffries JL, Willging JP, Cotton RT, et al. Left ventricular hypertrophy and abnormal ventricular geometry in children and adolescents with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165(10):1395-9.
13. Amin RS, Kimball TR, Kalra M, Jeffries JL, Carroll JL, Bean JA, et al. Left ventricular function in children with sleep disordered breathing. Am J Cardiol. 2005;95(6):801-4.
14. de la Eva RC, Baur LA, Donaghue KC, Waters KA. Metabolic correlates with obstructive sleep apnea in obese subjects. J Pediatr. 2002;140(6):654-9.
15. Waters KA, Mast BT, Vella S, de la Eva R, O'Brien LM, Bailey S, et al. Structural equation modeling of sleep apnea, inflammation, and metabolic dysfunction in children. J Sleep Res. 2007;16(4):388-95.
16. Kaditis AG, Alexopoulos EI, Damani E, Karadonta I, Kostadima E, Tsolakidou A et al. Obstructive sleep-disordered breathing and fasting insulin levels in nonobese children. Pediatr Pulmonol. 2005;40(6):515-23.
17. Santos AAC, Zanetta DMT, Cipullo JP, Burdmann EA. O diagnóstico da hipertensão arterial na criança e no adolescente. Pediatria (São Paulo). 2003;25(4):174-83.
18. Salgado CM, Carvalhaes JTA. Hipertensão arterial na infância. J Pediatr (Rio J). 2003;79(Suppl 1):S115-24.
19. Brodsky L, Moore L, Stanievich JF. A comparison of tonsillar size and oropharyngeal dimensions in children with adenotonsillar hypertrophy. Int J Pediatr Otorhinolaryngol. 1987;13(2):149-56.
20. Standards and indications for cardiopulmonary sleep studies in children. American Thoracic Society. Am J Respir Crit Care Med. 1996;153(2):866-78.
21. Kwok KL, Ng DK, Cheung YF. BP and arterial distensibility in children with primary snoring. Chest. 2003;123(5):1561-6.
22. Marcus CL, Greene MG, Carroll JL. Blood pressure in children with obstructive sleep apnea. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1098-103.
23. Li AM, Chan MH, Chan DF, Lam HS, Wong EM, So HK, et al. Insulin and obstructive sleep apnea in obese Chinese children. Pediatr Pulmonol. 2006;41(12):1175-81.
24. Tauman R, O'Brien LM, Ivanenko A, Gozal D. Obesity rather than severity of sleep-disordered breathing as the major determinant of insulin resistance and altered lipidemia in snoring children. Pediatrics. 2005;116(1):e66-73.
25. Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Disord. 2003;27(Suppl 3):S53-5.
26. Morrison JA, Friedman LA, Harlan WR, Harlan LC, Barton BA, Schreiber GB, et al. Development of the metabolic syndrome in black and white adolescent girls: a longitudinal assessment. Pediatrics. 2005;116(5):1178-82.
1. MSc at UNIFESP (MD, Otorhinolaryngologist).
2. MD, Otorhinolaryngologist (MD, Otorhinolaryngologist).
3. MSc at UNIFESP (MD, Otorhinolaryngologist).
4. PhD at UNIFESP (Assisting Physician at UNIFESP).
5. Post-Doctoral Degree at UNIFESP (Adjunct Professor at UNIFESP).
6. PhD at UNIFESP (Professor adjunto na UNIFESP).
UNIFESP.
Send correspondence to:
Daniele de Oliveira Soares Stefanini
Rua Itapeva, nº 500, 10º andar, conjunto D. Bela Vista
São Paulo - SP. CEP: 01332000
Paper submitted to the BJORL-SGP (Publishing Management System - Brazilian Journal of Otorhinolaryngology) on November 7, 2011.
And accepted on July 1, 2012. cod. 8889.