Year: 2012 Vol. 78 Ed. 1 - (13º)
Artigo Original
Pages: 81 to 86
pH salivary analysis of subjects suffering from Sjögren's Syndrome and laryngopharyngeal reflux
Author(s): Marco Antonio dos Anjos Corvo1; Claudia Alessandra Eckley2; Bianca Maria Liquidato3; Gustavo Leão Castilho4; Cibelle Nunes de Arruda5
Keywords: gastroesophageal reflux, saliva, laryngeal diseases, xerostomia.
Abstract:
Saliva is one of the components for the digestive homeostasis. Recent studies have shown that patients with laryngopharyngeal reflux (LPR) present a drop in salivary pH. Patients with Sjögren´s syndrome (SS) are a potential clinical research model for xerostomia and its laryngeal and pharyngeal consequences. The aim was to evaluate the characteristics of saliva of patients with SS and LPR.
METHODS: 19 patients with SS plus LPR, and 12 healthy controls had their saliva studied prospectively for volume and pH. Two salivary samples were obtained from each participant: whole unstimulated saliva(WUS) and whole stimulated saliva(WSS) while chewing parafilm M®. All the participants were females.
RESULTS: Mean age was 60 years (study group) and 44 years (control). LPR was diagnosed on all 19 subjects. The mean pH of WUS was 7.53 (SS) and 7.57 (controls), raising to 7.87 and 7.83 respectively after stimulation. The mean salivary volume of patients with SS was 1.27 mL (WUS) and 3.78 mL (WSS), whereas controls had a significantly higher salivary volume both before and after stimuli.
CONCLUSION: A very high prevalence of LPR was found in patients with SS, which is probably caused by a uniform drop in salivary volume and all its contents, rather than a specific deficiency in its components, as shown previously in patients without SS.
![]()
INTRODUCTION
Saliva plays a fundamental role in the digestive system's homeostasis, for its inorganic content - made up of water and bicarbonate ions, but primarily by its organic and protein make up1. Thus, one might question the clinical situation caused by xerostomia on the basic salivary functions of food bolus lubrication, on the beginning of the enzymatic digestion, buffering capacity and local immune response1. Previous studies indicate that low saliva volumes reduce the esophageal-salivary reflex effectiveness and could contribute to the occurrence of oral, esophageal and dyspeptic diseases2,3.
Xerostomia is defined as a subjective sensation of dry mouth (from the Greek xeros - dry stoma - mouth)4.5. It may be present in various diseases, among which we stress Sjögren's syndrome (SS)1,4-10. Of probable autoimmune origin, SS affects the exocrine glands, which undergo progressive lymphoplasmacytic infiltration and leads to functional failures9,10. Since the salivary glands are frequently affected by infiltration, the disease is recognized for causing clinically noteworthy xerostomia9,10.
Some authors have reported higher prevalence of gastroesophageal reflux disease (GERD) in populations with SS11-13. However, with regards to laryngopharyngeal reflux (LPR), only rare reports mention its occurrence as frequent in subjects with SS, with no specific studies designed to establish this correlation14-17.
For individuals with xerostomia, Eckley & Costa18 noticed significant drops in the pH and in the salivary volume of patients with LPR, when compared to normal individuals. Moreover, these changes seem to be a response to reflux, and not a primary deficiency, because after controlling the disease, pH and salivary volume go back to normal19. However, in SS individuals, it is not known how saliva could interfere in the balance of the laryngeal and esophageal coating epithelium. Would there be local tissue damage simply because of the volumetric reduction in salivary volume or would there be changes in the salivary make up involved in the genesis and maintenance of the findings?
Our hypothesis is that there would be some specific change in the saliva of individuals with SS which would cause a reduction in laryngeal and pharyngeal protection, making some of these individuals more susceptible to LPR, as it seems to happen with individuals without xerostomia18,20. The early identification of these individuals in risk by studying their salivary characteristics could be of high importance for preventing the complications associated with the laryngopharyngeal reflux, enhancing the quality of life of individuals with Sjögren's syndrome.
The present paper aims at comparing the salivary volume and pH of individuals with Sjögren's syndrome and laryngopharyngeal reflux to those of healthy individuals.
MATERIALS AND METHODS
After approval by the Ethics in Research with Human Beings' Committee of our institution (Project number 034/07), a total of 36 adult patients diagnosed with Sjögren were investigated in a cross-section manner. The patients were consecutively and randomly selected by telephone call, from the database of the stomatology wards of a tertiary university hospital, between January 2007 and December of 2009. We admitted all the patients who met the inclusion and exclusion criteria, after a statement about the objectives, methodologies and risks.
Inclusion factors were: adult patients with hyposalivation and a confirmed diagnosis of Sjögren's syndrome by the current diagnoses criteria of the American-European consensus21.
The exclusion factors were other conditions which could cause chronic laryngopharyngitis, such as: smoking, drinking alcoholic beverages, exposure to abrasive inhaling chemicals and overt allergic or infectious agents20. In addition, we took off the study those patients who were unable to produce a minimum amount of saliva for sampling and biochemical analysis, patients whom we could suspend prokinetic or proton pump inhibitor drugs, patients submitted to salivary gland surgery and patients with pre-neoplastic and neoplastic laryngeal and pharyngeal lesions (current or previously treated)18,20,22.
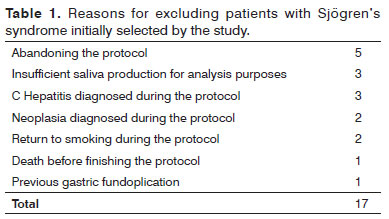
Of the initially 36 selected patients with SS by the criteria used, 17 were withdrawn from the study by the conditions described in Table 1.
The patients answered a detailed questionnaire about laryngopharyngeal and digestive symptoms suggestive of GERD and LPR, and subsequently underwent a thorough ENT examination and rigid laryngoscopy (without topical anesthesia). We used the flexible nasal-laryngoscope only in cases of hyperreflexia or impossibility to visualize the segment with the telescope.
Two consecutive samples of whole saliva were obtained from each participant: the first, unstimulated whole saliva (UWS) and second, stimulated whole saliva (SWS), mechanically by chewing a 25 cm2 piece parafilm M® (Pechiney Plastic Packing Chicago IL, USA)23.
Collection was always performed in the morning and with the patient fasting for at least eight hours. The patient was instructed not to brush his/her teeth or use mouthwash in the morning of saliva collection. The method of collection was the one previously described by Eckley et al.18-20
The UWS and SWS samples were collected by asking the patient to "spit" freely in a collection bottle the entire saliva volume produced during the 10-minute period. To preserve their rheological and biochemical characteristics, salivary samples were stored in a refrigerator at 5 degrees Celsius, until they could be processed and analyzed.
The processing was done in the same saliva collection period by centrifugation for 10 minutes (3500 rpm - Excelsa II centrifuge - Fanem - Brazil) for sedimentation of cellular debris; the supernatant was separated for pH and volume measurement. Salivary volume measurement was made using pipettes and graduated test tubes, being recorded in milliliters24. Salivary pH was measured using a digital pH meter (Denver Instrument Company, Model: Basic pH meter, Arvada, CO, USA).
The LPR diagnosis was made based on laryngeal signs and symptoms, using two research instruments, the rate of reflux symptoms (reflux symptom index - RSI)25, and the Reflux Finding Score (RFS)26, both previously validated in the literature for the English language25,26. Symptoms which added scores were equal to or higher than 13 in the RSI25 were considered positive for LPR, and laryngeal signs which added scores were higher than or equal to seven in the RFS26.
Laryngopharyngeal symptoms and direct laryngoscopy signs matched the results of endoscopy and/or dual-channel 24-hour esophageal pH monitoring.
With technical support from a specific professional, the results were plotted and analyzed using parametric and non-parametric statistical methods for variable analyses (Student's t test and Wilcoxon test). We considered a "p" value lower than or equal to 0.05 as significant.
To determine the importance of the results for the SS group examined we also studied a group of 12 healthy female volunteers, who were selected by following the same exclusion criteria set forth above. Moreover, none of the volunteers met the diagnostic criteria used for SS. All were also submitted to RSI25 and RFS26 and to rule out the presence of LPR, a condition for participating in the control group. Saliva collection and the salivary parameters studied were similar to those described for the study group.
RESULTS
All the 19 individuals in this study were females, with a mean age of 60 years (varying between 49 and 74 years). Nine patients had primary SS, seven cases were secondary to rheumatoid arthritis, two were secondary to systemic lupus erythematous and one to scleroderma. The mean diagnosis time for the individuals with SS at the time when the present study was analyzed was 58 months (4 years and 10 months), with a median of 5 years and 4 months and standard deviation of 2 years and 5 months.
The control group was made up of 12 healthy women, with mean age of 44 years (26 to 62 years), median of 42 years and standard deviation of 11.5; without laryngeal signs or symptoms of LPR. Mean RSI was zero and the mean RFS was 1.75, varying between zero and three points, both confirming that there was no posterior laryngitis.
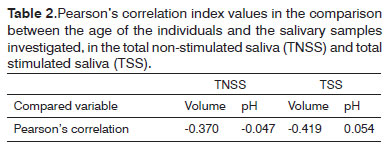
There was a difference between the mean age of the study group and that of the control group. Even then, when we correlated the age of all the individuals (study and control groups) with the quantitative salivary variables studied (volume and pH), the Pearson's correlation index showed low values, indicating that both groups could be compared vis-à-vis these variables (Table 2).
In the study group, the mean RSI was 19.5, varying between 13 and 30 points. The mean RFS was 11.56, varying between eight and eighteen points.
Of the eight patients with reflux esophagitis in the study group, four had non-erosive esophagitis and four and the erosive type (Los Angeles grade A), there was no one with Barret's esophagus in the series. Because they had RSI and RFS suggestive of laryngopharyngeal reflux, with UGE (upper gastrointestinal endoscopy) confirming gastroesophageal disease, in eight individuals we confirmed the laryngopharyngeal reflux. In the remaining 11 individuals in whom UGE was not changed, we did manometry and esophageal pH measuring, according to the methodology utilized.
As to the esophageal manometry, seven patients had a normal exam, two patients had hypotonia of the lower esophageal sphincter, and two had mild esophageal dysmotility. However, as to the double-sensor esophageal pH probe study, the result was uniform, because all the 11 patients in the study group had pathological reflux episodes in the proximal sensor, making up the LPR diagnosis. Only three of these individuals had reflux in pathological levels also in the distal sensor, which configures GERD and LPR together.
When we analyzed the individuals with SS as to disease subtype (primary or secondary), we did not notice a statistically significant difference, by the Mann-Whitney test, in characterizing the sample as to age (p=0.549), as to RSI (p=0.447) and as to RFS (p=0.400). Since the SS subtypes were statistically equal for the salivary variables analyzed (volume and pH), we considered the individuals with SS in the same group in order to compare them with the control group.
Salivary pH and volume results are presented on Figures 1 and 2, and are described below.
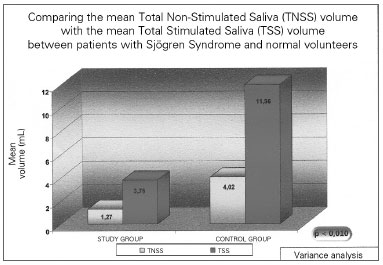
Figure 1. Salivary mean volume in individuals with Sjögren (study group) and in healthy individuals - TNSS- Total Non-Stimulated Saliva TSS - Total Stimulated Saliva.
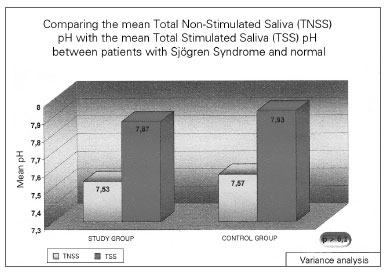
Figure 2. Mean salivary ph in individuals with Sjögren (study group) and in healthy individuals - TNSS - Total Non-Stimulated Saliva TSS- Total Stimulated Saliva.
The total unstimulated saliva volume (TUSV) of the individuals with SS varied between 0.1 and 4.1 mL (mean value of 1.27 mL) (sd=1.06). The mean Total Stimulated Saliva (TSS) volume was 3.78 mL, varying between 0.1 and 10.3 mL (sd=2.87). This difference was statistically significant (p=0.009). TUSV pH in patients with SS showed a mean value of 7.53, varying between 6.40 - 8.40 (sd=0.51), and TSS was 7.87, varying between 6.67 and 8.43 (sd=0.47), and this difference was also statistically significant (p =0.002).
In the group of healthy volunteers, the mean salivary volume of the NSSV was 4.02, varying between 1.6 and 8.0mL, and the STS was 11.96 mL, varying between 4.5-19.0mL (p=0.002). Now, the mean NSTS pH was 7.57, varying between 6.83 and 7.92; and the mean value of the TSS was 7.93, varying between 7.56 and 8.17 (p=0.004). All the above-studied variables in the control group showed statistically significant differences between the stimulated and the non-stimulated salivary samples.
As expected, SS patients had a statistically lower NSTS and STS mean volumes when compared to the control individuals (p<0.001). Nonetheless, there was no statistically significant difference in the saliva pH between the study and the control groups, both for the total non-stimulated and the stimulated total saliva (p>0.1).
DISCUSSION
The motivation to go more in-depth in understanding this topic stemmed from the need to understand LPR genesis more in-depth, and in the empirical observation of some patients with LPR being followed up, who had hyposalivation, according to the studies carried out by Eckley, in 200419. Thus, the project was conceived to conjugate the research objectives in salivary biochemical factors in the LPR genesis, with the need to offer our SS patients a better understanding of their disease and all the manifestations surrounding them.
For the LPR diagnosis, we considered the laryngoscopic signs and symptoms translated by the RSI25 and RFS26 instruments, respectively; nonetheless in association with the UDTE, esophageal pressure and 24-hour double channel esophageal pH probe data. We agree with the current trend that the RFS seems to be a good index to assess the presence of endoscopic signs of laryngitis, without this laryngeal inflammation being necessarily linked to reflux of the gastroduodenal content such as etiology (reason to adopt such strict exclusion criteria)17,22. Thus, the present study agrees with the recent publications by Gupta & Sataloff17 and Ali22 as admitting to a suspicion of LPR only when the RFS values higher than seven were associated with characteristic symptoms, with the RSI greater than 13 which still relates to the patient's clinical complaint as an important suspicion for diagnosis. Even if ideal, the conjugation of these two instruments was paramount to establish the LPR suspicion, which was later corroborated by UDTE and the double channel 24 hour esophageal pH probe.
Although rare, the literature had already considered them possible laryngeal manifestations of the SS. In a 2003 publication, Belafsky & Postma11 empirically recognized a high prevalence of LPR and esophageal dysmotility in patients with SS11. In the current series, only two of the 19 patients with SS had esophageal dysmotility.
In 2005, Ogut et al.12, submitted 77 patients with SS to RSI and RFS search criteria, concluding that in this subgroup, the score values were significantly higher when compared to the normal individuals studied. The present study achieved great notability because of the expressive series reported, and it also stimulated the current study to try to understand the reason for such a high referred association.
Therefore, considering the previous literature data, and considering the protective properties of saliva to the digestive system18,20, we expected to find laryngopharyngeal changes in patients with SS. The initial hypothesis was that the association with SS with LPR would happen because of qualitative and not quantitative salivary deficiency. However, the current series did not allow to establish a correlation, since all the patients studied had LPR.
Analyzing the salivary data, we noticed that the mean volume of the TNSS samples from patients with SS was really low (1.27mL), with pH values slightly alkaline (mean value of 7.53). Even if these patients suffer from a saliva-production-limiting disease, the masticatory stimulation employed was able to increase the saliva volume (mean of 3.78mL), with a statistically significant difference. The mean TSS pH value went up to 7.87, reflecting a greater alkalinity of the fluid after the stimulus. This pH increase is particularly relevant for SS patients, if we consider the large prevalence of LPR seen in the study and the need for these patients to, theoretically, having greater efficiency in salivary buffering.
Since all SS patients had a behavior similar to that of LPR, comparing TNS and TSS variables of individuals with SS and without LPR were fundamental in the attempt to interpret the findings.
In relation to the results from the volumes obtained, the TNSS volume from SS individuals was reduced, showing a mean value of 1.27mL in a 10-minute collection. This value was statistically lower when compared to that from the control group, as to be expected for populations with SS.
About the pH of the salivary samples, it was noticed that the masticatory stimulus did not cause a statistically significant difference within the groups analyzed (control and study). Moreover, when compared to healthy individuals, the salivary samples of individuals with SS who had statistically equal pH values. Thus, differently than patients with LPR and without xerostomia, who had more acid saliva pH values in comparison with normal individuals, the patient with SS and with LPR did not have this altered mean pH value19,20,27. In other words, changes in salivary pH did not seem to be involved in the greater occurrence of LPR in patients with SS, suggesting a different pathophysiology from the LPR for this special group.
The rarity of SS in the world and the lack of official data both on disease prevalence as to its other particularities in Brazil make the recording of the reflux behavior in this subpopulation a topic very little explored before9,10. The results presented in this study represent part of an ongoing study about the organic and inorganic characteristics of patients with SS, and its influence in the genesis of LPR and GERD. Thus, greater details on the pathophysiology of the disease in this subgroup of patients with SS are awaited, as new data become available.
CONCLUSIONS
Based on the present study, we concluded that there was no statistically significant difference in the salivary pH of individuals with Sjögren syndrome and healthy individuals, both in the non-stimulated total saliva as well as in the total stimulated saliva. This suggests that the laryngopharyngeal reflux may have a unique pathophysiology in SS individuals, possibly associated with the global drop in salivary volume and all its components.
REFERENCES
1. Boyce HW, Bakheet MR. Sialorrhea: a review of a vexing, often unrecognized sign of oropharyngeal and esophageal disease. J Clin Gastroenterol. 2005;39(2):89-97.
2. Sarosiek J, McCallum RW. What role do salivary inorganic components play in health and disease of the esophageal mucosa? Digestion. 1995;56(Suppl 1):24-31.
3. Mason DK, Chisholm DM. Salivary Glands in health and disease. Philadelphia: Saunders; 1974.
4. Camargo ACK, Pupo DB, Bussoloti Filho I. Sialometria. Acta ORL/Técnicas em Otorrinolaringologia. 2005;23(1):14-8.
5. Korn GP, Pupo DB, Quedas A, Bussoloti Filho I. Correlação entre o grau de xerostomia e o resultado da sialometria em pacientes com Síndrome de Sjögren. Rev Bras Otorrinolaringol. 2002;68(5):624-8.
6. Sreebny LM, Valdini A, Yu A. Xerostomia. Part II: Relationship to nonoral symptoms, drugs, and diseases. Oral Surg Oral Med Oral Pathol. 1989;68(4):419-27.
7. Mathews SA, Kurien BT, Scofield RH. Oral manifestations of Sjögren's syndrome. J Dent Res. 2008;87(4):308-18.
8. Sood S, Anthony R, Pease CT. Sjögren's syndrome. Clin Otolaryngol Allied Sci. 2000;25(5):350-7.
9. Liquidato BM. Avaliação da sialometria e biópsia de glândula salivar menor na classificação de pacientes com Síndrome de Sjögren. Departamento de Otorrinolaringologia. São Paulo: Faculdade de Ciências Médicas da Santa Casa de São Paulo-2004.
10. Felberg S, Dantas PE. Sjögren's syndrome: diagnosis and treatment. Arq Bras Oftalmol. 2006;69(6):959-63.
11. Belafsky PC, Postma GN. The laryngeal and esophageal manifestations of Sjögren's syndrome. Curr Rheumatol Rep.2003;5(4):297-303.
12. Ogut F, Midilli R, Oder G, Engin EZ, Karci B, Kabasakal Y. Laryngeal findings and voice quality in Sjögren's syndrome. Auris Nasus Larynx. 2005;32(4):375-80.
13. Ramos HV, Pillon J, Kosugi EM, Fujita R, Pontes P. Laryngeal assessment in rheumatic disease patients. Braz J Otorhinolaryngol. 2005;71(4):499-503.
14. Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope.1991;101(4 Pt 2 Suppl 53):1-78.
15. Richter JE. Extraesophageal presentations of gastroesophageal reflux disease. Semin Gastrointest Dis. 1997;8(2):75-89.
16. Koufman JA, Amin MR, Panetti M. Prevalence of reflux in 113 consecutive patients with laryngeal and voice disorders. Otolaryngol Head Neck Surg. 2000;123(4):385-8.
17. Gupta R, Sataloff RT. Laryngopharyngeal reflux: current concepts and questions. Curr Opin Otolaryngol Head Neck Surg. 2009;17(3):143-8.
18. Eckley CA, Costa HOO. Estudo da concentração salivar do fator de crescimento epidérmico em indivíduos com laringite crônica por refluxo laringofaríngeo. Rev Bras Otorrinolaringol. 2003;69(5):590-7.
19. Eckley CA, Rios LS, Rizzo LV. Estudo comparativo da concentração salivar do fator de crescimento epidérmico em indivíduos com laringite crônica por doença do refluxo gastroesofágico antes e após o tratamento: resultados preliminares. Braz J Otorhinolaryngol. 2007;73(2):156-60.
20. Eckley CA, Michelsohn N, Rizzo LV, Tadokoro CE, Costa HO. Salivary epidermal growth factor concentration in adults with reflux laryngitis. Otolaryngol Head Neck Surg. 2004;131(4):401-6.
21. Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61(6):554-8.
22. Ali Mel-S. Laryngopharyngeal reflux: diagnosis and treatment of a controversial disease. Curr Opin Allergy Clin Immunol. 2008;8(1):28-33.
23. van der Reijden WA, Van der Kwaak JS, Veerman EC, Nieuw Amerongen AV. Analysis of the concentration and output of whole salivary constituents in patients with Sjögren's syndrome. Eur J Oral Sci. 1996;104(4( Pt 1)):335-40.
24. Marcinkiewicz M, Han K, Zbroch T, Poplawski C, Gramley W, Goldin G, et al. The potential role of the esophageal pre-epithelial barrier components in the maintenance of integrity of the esophageal mucosa in patients with endoscopically negative gastroesophageal reflux disease. Am J Gastroenterol. 2000;95(7):1652-60.
25. Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice. 2002;16(2):274-7.
26. Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS). Laryngoscope. 2001;111(8):1313-7.
27. Costa HOO, Eckley CA. Correlação do pH e volume salivares com sintomas laringofaríngeos. Rev Bras Otorrinolaringol. 2004;70(1):24-8.
1. MSc in Medicine - Medical Sciences School - Santa Casa de São Paulo (Second Assistant Professor - Department of Otorhinolaryngology of the Santa Casa de Misericórdia hospital of São Paulo).
2. PhD in Medicine - Medical Sciences School - Santa Casa de São Paulo (Assistant Professor - Department of Otorhinolaryngology of the Santa Casa de Misericórdia hospital of d São Paulo).
3. PhD in Medicine - Medical Sciences School - Santa Casa de São Paulo (Assistant Professor - Department of Anatomy and Physiology of the Santa Casa de Misericórdia hospital of São Paulo).
4. 2nd Year Resident in Otorhinolaryngology - Santa Casa de Misericórdia de São Paulo.
5. 1st Year Resident in Otorhinolaryngology - Santa Casa de Misericórdia de São Paulo.
Departamento de Otorrinolaringologia da Faculdade de Ciências Médicas da Santa Casa de São Paulo.
Send correspondence to:
Marco Antonio dos Anjos Corvo
Alameda Joaquim Eugenio de Lima, 1601 - Ap 141, Jardim Paulista
São Paulo - SP, Brasil. Zip Code: 01403-003
Paper submitted to the BJORL-SGP (Publishing Management System - Brazilian Journal of Otorhinolaryngology) on March 05, 2011
Accepted on July 28, 2011. cod. 7622