Year: 2010 Vol. 76 Ed. 1 - (18º)
Artigo Original
Pages: 107 to 112
Maternal and paternal age, birth order and interpregnancy interval evaluation for cleft lippalate
Author(s): Daniella Reis Barbosa Martelli1, Kaliany Wanessa da Cruz2, Letízia Monteiro de Barros3, Marise Fernandes Silveira4, Mário Sérgio Oliveira Swerts5, Hercílio Martelli Júnior6
Keywords: cleft lip, cleft palate, maternal age, paternal age, birth order.
Abstract:
Cleft lip and palate (CL/P) are the most common congenital craniofacial anomalies. Aim: To evaluate environmental risk factors for non-syndromic CL/P in a reference care center in Minas Gerais. Materials and Methods: we carried out a case-controlled study, assessing 100 children with clefts and 100 children without clinical alterations. The analysis dimensions (age, skin color, gender, fissure classification, maternal and paternal age, birth order and interpregnancy interval), obtained from a questionnaire; and later we build a data base and the analyses were carried out by the SPSS 17.0 software. The results were analyzed with the relative risk for each variable, in order to estimate the odds ratio with a 95% confidence interval, followed by a bivariate and multivariate analysis. Results: among 200 children, 54% were males and 46% were females. As far as skin color is concerned most were brown, white and black, respectively. Cleft palates were the most common fissures found (54%), followed by lip cleft (30%) and palate cleft (16%). Conclusion: although with a limited sample, we noticed an association between maternal age and an increased risk for cleft lip and palate; however, paternal age, pregnancy order and interpregnancy interval were not significant.
![]()
INTRODUCTION
Neural tube defects and orofacial fissures are among the most common congenital alterations1. Non-syndromic cleft lip and palate (CLP) are the most common anomalies in the skull-facial area (OMIM 119530). In many regions of the world, CLP is more common than the Down syndrome1.
Every two minutes, a child with CLP is born in the world, 660 children daily and 235 thousand new cases of fissures are seen annually. With the growth of the world population, we expect 3,200 new cases per year of CLP2.
The incidence of CLP varies according to geographic location, race and socio-economic condition3. Fogh- Andersen4 reported an incidence of 1.5 cases of CLP for every 1,000 births in Denmark, while in other regions the occurrence varied (1-2.69:1,000)1,5. Recently, Martelli-Júnior et al.6 reported an incidence of 1.46 fissures for every 1,000 births, in the state of Minas Gerais, Brazil. Studies show that the Asian population, ancestors of Native Americans and Northern Europeans have a higher incidence of CLP7. In contrast, Africans and descendants have a greater incidence of lip fissure8.
As far as embryology is concerned, CLPs result from primary defects on the craniofacial fusion which form the primary and secondary palates, in the first trimester of the intrauterine development9. These clinical fissures may be classified, having the incisive foramen as anatomical basis, in four groups: pre-incisive foramen or cleft lip (CL), post-incisive foramen fissures or cleft palate (CP), transincisive foramen fissures or cleft lip and palate (CLP) and rare facial fissures10.
Together, the CLP make up a heterogeneous group of alterations, having a multifactorial origin, other genetic and environmental factors contribute to their etiology. It is greatly important to identify the etiological factors associated with a disease, because by knowing them it is possible to enhance our understanding of the disease and better develop prevention measures11. Among the environmental risk factors for CLP we stress: smoking, maternal and paternal age, alcohol, birth order, interpartum interval and folic acid deficiencies8,11. The goal of the present study was to evaluate, because of the lack of Brazilian studies, the association or not of environmental risk factors, especially maternal and paternal age, birth order and interpartum interval with CLP.
MATERIALS AND METHODS
We carried out a case-control study in order to assess the environmental risk factors associated with CLP, in a multiprofessional Reference Service for craniofacial deformities in the state of Minas Gerais, Brazil, between 2006-2008. The population in this study was made up of 200 children with and without non-syndromic CLP from similar socio-economic backgrounds. The group "case" had 100 parents from children (aged between 0 and 12 years), with non-syndromic CLP, diagnosed and being rehabilitated in the aforementioned institution, regardless of gender, skin color, place or country of birth. The "control" group had 100 parents of children in the same age range (between 0 and 12 years); however, without clinical alterations or craniofacial defects, seen at the odontopediatrics department of the same institution. Both groups had similar socio-economic backgrounds; and also the other inclusion criteria were similar. The aforementioned Reference Center treats exclusively patients from the public health care system (SUS), being certified by the Ministry of Health. From both groups we excluded the parents who did not accept to participate in the study, children with syndromic CLP and parents from consanguineous marriages.
In order to assess the risk factors, in both groups we used an individual instrument (guided questionnaire), encompassing the following analysis aspects: age, skin color, gender, cleft type, parents' ages, birth order and interpartum interval. The questionnaires were deployed always by the same examiners (DRBM and KWC) after being properly trained for the activity. The questionnaire was answered by both groups always after the clinical visit, with the mothers, thus avoiding any harm to the patients in terms of consultation and clinical visit. Each questionnaire was answered in one single visit. Previously, we held a pilot study to assess and check examiners' calibration and the very feasibility of the data collection instrument.
In the "case" group, the non-syndromic CLP cases were classified having the incisive foramen as anatomical reference10 in: (1) CL: including complete or incomplete, uni or bilateral pre-foramen clefts; (2) CLP: including unilateral and bilateral transforaminal clefts and pre and post-foramen clefts; (3) CP: include all the complete and incomplete post-foramen clefts and (4) Others: the rare facial clefts.
After deploying the questionnaires, the information collected was filed in a data bank and analyzed by the SPSS version 17.0 (Chicago, EUA) statistical software. The data was analyzed with relative risk for each variable in order to estimate the odds ratios (OR) with a 95% confidence interval, followed by a bivariate and multivariate analysis. This study was led according to the guidelines established by Ordinance 196/88 from the National Health Council - Ministry of Health, and it was submitted to and approved by the Ethics Committee of the University. Each participant in the study signed a Free and Informed Consent Form.
RESULTS
In the "case" group, of the 100 children with CLP, 64 (64%) were males and 36 (36%) were females, while in the "control" group, 56 (56%) were females and 44 (44%) males. As far as skin color is concerned, among the cases, 64%, 30% and 6% were brown, white and black, respectively; while among the controls, 45%, 32% and 23% were, brown, white and black, respectively. Table 1 shows, in both groups, the children distribution according to the different ages. We see that among the "cases" there was a prevalence of children between 0 and 3 years, representing 44% in this group; while in the "control" group there was a greater prevalence of children between 7 and 9 years, representing 44% in this group. Thus, when both groups are considered together, we see a predominance of children between 7 and 9 years of age.
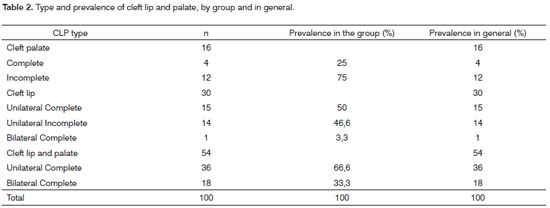
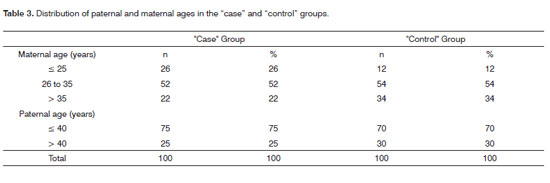
Table 2 shows the prevalence of CLP seen among the participants of the study, as well as their percentage values in the general distribution and distribution by group. We see that there were no cases of rare clefts in this population. The most common among the clefts studied was the CLP (54%), followed, respectively by cleft lip (30%) and cleft palate (16%). Table 3 shows the distribution of maternal and paternal ages in children with CLP, by age range. We see that both among "cases" and "controls" there was a greater prevalence of mothers between 26 and 35 years. In the age range up to 25 years, the "cases" had a greater number of mothers, when compared to the "controls" (26% and 12%, respectively), and such distribution was inverted in women older than 35 years, where there were more mothers in the "control" group when compared to "cases" (34% and 22%, respectively). As far as paternal age is concerned, we see that it was broken down in intervals up to 40 years and above 40 years. Both in the group "case" and in the "control" group we observed a frequency of up to 40 years, respectively 75% and 70%.
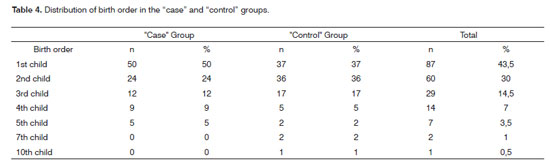
In relation to the number of pregnancies, in both groups we noticed that among "cases" 39% of the mothers had 1 gestation, 29% (2 gestations) and 15% with 3 gestations. We still found 8% of the mothers who had 4 gestations, 6% with 5 gestations and 3% with 6 gestations. Among "controls", 2 gestations were more frequent (39%), 3 (24%), 1 (18%) and 4 gestations were less observed (10%), 5 (6%), 8 (2%) and 6 (1%). As far as birth order is concerned, Table 4 shows the distribution both in the "cases" group as well as in the "control" group of children with CLP. We observed that 74% of the children with clefts were born in the first two generations. There were no statistical differences between the groups in relation to birth order (OR: 0.6; 95% CI: 0.34-1.05). All the information associated with the birth order was established considering 1, 2, 3, 4 or more gestations for individuals with CLP and in the "control" group. Category "1" was considered without risk for CLP (OR=1).
Table 5 shows the use of a bivariate analysis in relation to size, maternal and paternal ages and interpartum interval. We have also seen that maternal age showed statistical significance (p<0.05), which was also observed in the logistical regression (multivariate analysis). When the maternal and paternal ages were additionally statistically treated there was no statistical significance (p<0.05). The interpartum interval was distributed in the following chronological intervals: 1 to 24 months, 25 to 48 months and above 48 months. We did not observe statistical differences between the "cases" and "controls" and the interpartum intervals.
DISCUSSION
Different epidemiological studies have been carried out in order to assess CLP distribution12,13. It is accepted that the different types of clefts with distinct distribution and the incidences vary among the different populational groups14. Notwithstanding, Orientals, Native Americans, Australian Aborigines and Northern Europeans represent the most affected populations; while Africans and African descendants are more affected by isolate CP8. In the present study, of the 100 clefts assessed, the most commonly found was the CLP, making up 54% of the ones observed. Following the CLP, the CL alone represented 30% and the CP made up 16% of the population studied. A study assessing 126 Brazilian children with non-syndromic CLP showed a prevalence of 2.57 times higher of CLP in males, when compared to females. This same study showed a greater occurrence of CLP followed, respectively of CL and CP alone. Recently, a study carried out in the same institution where this study was held, showed were similar results to the ones hereby presented16.
Maternal age is considered a risk factor for numerous chromosomal alterations; however, there is no consensus whether or not it represents a risk factor for CLP. Most of the studies prior to 1970 suggested an association between CLP and maternal age17. As of 1970, many papers were published showing conflicting results17. A study carried out in California showed that women older than 39 years had twice the risk of having a child with CLP when compared to mothers between 25 and 29 years18. Another study with Americans, residents of San Francisco found an association between CP and young women19. The epidemiological analysis with a Chinese population showed a relationship between advanced maternal age and bilateral CLP among males and CLP among females20. Nonetheless, studies carried out in Canada, Iran, Holland and South America did not show association between maternal age and CLP17. The results from the present study showed that, although with a limited population of patients with CLP, maternal age was significant in relation to the occurrence of clefts - using the OR analysis and a 95% confidence interval (Table 5). We noticed that the temporal intervals were from 26 to 35 years and higher than 35 years had reduced risk of having CLP when compared to women with ages lower than 26 years. In a Meta analysis done by Vieira et al.17, they did not report any general association between maternal age and CLP. There was a relation between CP and women between 20 and 24 years and above 30 years. One important confounding factor in this study can be the race profile of the populations evaluated. A difficulty in assessing the subjects' social and economic backgrounds, especially in different populations and in different countries; which can explain the differences in the populations studied. In the present study, the population evaluated was from the State of Minas Gerais - a mix of Europeans (mostly Portuguese and Italians), Africans and Native South Americans in a smaller percentage.
Another confounding factor of maternal age is paternal age. It is well known that advanced paternal age (> 40 years) is associated with a risk increase in different diseases, such as achondroplasia, Apert Syndrome and Neurofibromatosis, and a relation with CLP is possible17. Association of mutations in MSX1 (muscle segment homeobox) and CLP corroborates the hypothesis of the relation between paternal age and CLP, which has been observed in the Apert syndrome8. Hay21 and Bille et al.22 reported that the risk of CLP with increased maternal age can be reflected in the increase in paternal age. In the present study paternal age did not show statistically significant association with CLP (OR: 0.7; 95% CI: 0.36-1.35) (Table 5). We also did not observe statistical significance when we assessed maternal and paternal ages associated with CLP risk.
There is evidence showing an association between birth order (children of later gestations) and diseases such as: type 1 diabetes, schizophrenia and breast cancer23. There is also an association between birth order and congenital heart diseases, neural tube defects and CLP20. Nonetheless, there is no consensus whether birth order has any association with CLP23. In the present study there was no significant statistical association between these variables (OR: 0.6; 95% CI: 0.34-1.05). Among CLP patients, 74% were born from the first two gestations. Even when the statistical analysis was carried out in the group of patients with CLP, there was no association between birth order and CLP (x2=0.76; p=0.383). One of the first case-control studies carried out to check the association between birth order and CLP was held in 1953, in England, and they did not report correlations between the two conditions24. Later on, evaluations carried out in populations from Sri Lanka, France and Iran also did not report associations between birth order and CLP25-27. On the other hand, investigations carried out in Latin America, Switzerland, USA and China showed positive associations between birth order and CLP20,28,29. Vieira and Orioli23, did a Metanalysis on the order of birth and CLP and showed a positive statistical association between the conditions.
Scientific investigations have shown an association between a short interpartum interval (< 6 months) and many alterations, including neural tube defects, congenital heart diseases, low birth weight babies and anemia11. Such association has been assigned to a reduction in the levels of folic acid30. There are indications that a supplementation with folic acid may have a protective effect for numerous congenital defects, including CLP31. Considering these alterations, especially folic acid supplementation, has proved a reduction of 1/3 in the risk of CLP32. Reductions in the levels of folate seen in reduced interpartum intervals have been associated to an increased risk of fetal growth restriction33. Recently, it was shown that maternal obesity and longer interpartum intervals are associated with CP34. In the present investigation, the interpartum periods were classified in three intervals, and we did not observe statistically significant differences among them (Table 5).
As to the pathogenesis of CLP, Vieira9 compared this biological event with a jigsaw puzzle with more than 100 pieces and that many genes (between 3 and 14), plus some risk factors involved in the origin of CLP. Thus, although we understand better the participation of genes such as the IRF6 (Interferon 6 Regulatory factor), FGF (fibroblastic growth factor), MSX1 and environmental risk factors, the practical application of such knowledge is still limited. Thus, studies to better understand the action of these agents in animal models and in vitro will enable us to develop more efficient therapeutic tests in the future.
CONCLUSION
In this case-controlled study we assessed the environmental risk factors associated with non-syndromic CLP and we observed that there was a predominance of CLP, CL and CP alone, respectively. Although with a limited population coming from only one Brazilian state, we noticed an association between maternal age and increased risk for CLP. We also noticed a temporal interval of 26 to 35 years and above 35 years with a reduced risk for CLP compared to women younger than 25 years of age. Notwithstanding, paternal age, birth order and interpartum interval were not statistically significant for the occurrence of CLP.
Acknowledgement: We thank the patients and family members who participated in the study. We appreciate the suggestions from Dr. Antônio Prates Caldeira. We thank the Research Support Foundation of the State of Minas Gerais (Fapemig) and the National Council for Scientific Development (CNPq) (HMJ).
REFERENCES
1. OPCS Congenital malformation statistics. Notifications 1992. London: HMSO; 1995.
2. Kot M, Kruk-Jeromini J. Analysis of family incidence of cleft lip and/ or palate. Med Sci Monit. 2007;13(3):231-4.
3. Cobourne MT. The complex genetics of cleft lip and palate. Eur J Orthod. 2004;26(4):7-16.
4. Fogh-Andersen P. Inherance of harelip and cleft palate: contribution to the elucidation of the etiology of the congenital clefts of the face 1942 [Dissertation]. Copenhagen: Busck; 1942.
5. Tolarová MM, Cervenka J. Classification and birth prevalence of orofacial clefts. Am J Med Genet. 1998;75(2):126-37.
6. Martelli-Júnior H, Orsi-Júnior J, Chaves MR, Barros LM, Bonan PRF, Freitas JAS. Estudo epidemiológico das fissuras labiais e palatais em Alfenas, Minas Gerais, de 1986 a 1998. Rev Fac Odontol Univ São Paulo. 2006;13(1):31-5.
7. Mossey PA, Little J. Epidemiology of oral clefts: an international perspective. In cleft lip & palate. From origin of treatment. New York: Oxford University Press; 2006. p. 127-58.
8. Vieira AR. Unraveling human cleft lip and palate research. J Dent Res. 2008;87(2):119-25.
9. Meng L, Bian Z, Torensma R, Van der Hoff JW. Biological mechanisms in palatogenesis and cleft palate. J Dent Res. 2009;88(1):22-33.
10. Spina V, Psillakis JM, Lapa FS, Ferreira MC. Classificação das fissuras lábio-palatinas. Rev Hosp Clin Fac Med S Paulo. 1972;27(2):5-6.
11. Zeiger JS, Beaty TH. Is there a relationship between risk factors for oral clefts? Teratology. 2002;66(3):205-8.
12. Derijcke A, Eerens A, Carels C. The birth prevalence of oral clefts: a review. Br J Oral Maxillofac Surg. 1996;34(2):488-94.
13. Vanderas AP. Birth prevalence of cleft lip, cleft palate and cleft lip and palate among races: a review. Cleft Palate J. 1987;24(5):147-53.
14. Wantia N, Rettinger G. The current understanding of cleft lip malformations. Facial Plast Surg. 2002;18(4):147-53.
15. Martelli-Junior H, Porto LCVP, Barbosa DRB, Bonan PRF, Freitas AB, Coletta RD. Prevalence of nonsyndromic oral clefts in a reference hospital in Minas Gerais State, between 2000-2005. Braz Oral Res. 2007;21(4):314-17.
16. Paranaíba LMR, Almeida H, Barros LM, Martelli DRB, Orsi-Júnior JD, Martelli-Júnior H. Técnicas cirúrgicas correntes para fissuras lábiopalatinas, em Minas Gerais, Brasil. Braz J Otorhinolaryngol. 2009, in press.
17. Vieira AR, Orioli IM, Murray JC. Maternal age and oral clefts: A reappraisal. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94(5):530-5.
18. Shaw GM, Croen LA, Cury CJ. Isolated oral cleft malformations: associations with maternal age and infant characteristics in a California population. Teratology. 1991;43(2):225-8.
19. Savitz DA, Schwingl PJ, Keels MA. Influence of paternal age, smoking, and alcohol consumption on congenital anomalies. Teratology. 1991;44(3):429-40.
20. Cooper ME, Stone RA, Liu YE, Hu DN, Melnick M, Marazita ML. Descriptive epidemiology of nonsyndromic cleft lip with or without cleft palate in Shangai, China, from 1980 to 1989. Cleft Palate Craniofac J. 2000;37(3):274-80.
21. Hay S. Incidence of clefts and parental age. Cleft Palate J. 1967;4(1):205-13.
22. Bille C, Skytthe A, Vach W, Knudsen LB, Andersen AMN, Murray JC, Christensen K. Parent's age and the risk of oral cleft. Epidemiology. 2005;16(3):311-6.
23. Vieira AR, Orioli I. Birth order and oral clefts: a meta analysis. Teratology. 2002;66(4):209-16.
24. Macmahon B, mckeown T. The incidence of harelip and cleft palate related to birth rank and maternal age. Am J Hum Genet. 1953;5(2):176-83.
25. Aramatunga NAS. A study of etiologic factors for cleft lip and palate in Sri Lanka. J Oral Maxillofac Surg. 1989;47(4):7-10.
26. Stoll C, Alembik Y, Dott B, Roth MP. Epidemiological and genetic study in 207 cases of oral clefts in Alsance, northeastern France. J Med Genet. 1991;28(5):325-9.
27. Rajabian MH, Sherkat M. An epidemiologic study of oral clefts in Iran: analysis of 1,669 cases. Cleft Palate Craniofac J. 2000;37(7):191-6.
28. Menegotto BG, Salzano FM. Epidemiology of oral clefts in a large South American sample. Cleft Palate Craniofac J. 1991;28(4):373-7.
29. Robert E, Kallen B, Harris J. The epidemiologic of orofacial clefts. J Craniofac Genet Dev Biol. 1996;16(2):234-41.
30. Oneill J. Do folic acid supplements reduce facial clefts? Evid Based Dent. 2008;9(3):82-3.
31. Czeizel AE, Toth M, Rockenbauer M. Population-based case control study of folic acid supplementation during pregnancy. Teratology. 1996;53(4):345-51.
32. Villamor E, Sparén P, Cnattingius S. Risk of oral clefts in relation to prepregnancy weight change and interpregnancy interval. Am J Epidemiol. 2008;167(11):1305-11.
33. Van Eijsden M, Smits LJ, van der Wal MF, Bonsel GJ. Association between short interpregnancy intervals and term birth weight: the role of folate depletion. Am J Clin Nutr. 2008;88(1):147-53.
34. Wilcox AJ, Lie RT, Solvoll K, Taylor J, mcconnaughey DR, Abyholm F. Folic acid supplements and risk of facial clefts: national population based care case-control study. BMJ. 2007;334(4):464.
1. MSc, Professor of Semiology - State University of Montes Claros - Unimontes.
2. Dentist - Scientific Initiation Scholarship Holder - CNPq.
3. PhD, Professor - University of Alfenas.
4. MSc. Professor - Graduate Program in Health Sciences - State University of Montes Claros -Unimontes.
5. PhD, Professor - University of Alfenas.
6. PhD, Full Professor.
Paper submitted to the BJORL-SGP (Publishing Management System - Brazilian Journal of Otorhinolaryngology) on April 20, 2009;
and accepted on September 9, 2009. cod. 6365