Year: 2003 Vol. 69 Ed. 2 - (12º)
Artigo Original
Pages: 222 to 225
Otoacoustic emissions x Cisplatin: precocious detection of ototoxicity in oncologic patients
Author(s):
Ruysdael Zocoli[1],
Sandro Laércio Reichow[2],
Ângela Maria Fontana Zocoli[3]
Keywords: hearing, otoacoustic emissions, ototoxicity, cisplatin.
Abstract:
Introduction: Studies show that there are numerous substances known as potentially ototoxic and among the main ones in the medicine classes, stands out the chemotherapeutics. Objective: The objective of this work was to achieve a study of the ototoxicity through otoacoustic emissions - product of distortion (EOA - PD), pre and post-application of the chemotherapeutic Cisplatin, in adults individuals, residents of the city of Blumenau/SC. Study Form: Clinic prospective. Material and Method: There were 11 individuals studied with oncologic pathology, age between 29 and 73 years old, through the otoacoustic emissions - product of distortion, previous and subsequently to the Cisplatin application, using the Madsen Cappela equipment coupled to the notebook Toshiba satellite computer. Results: The results didn't show significant changes on the answers of the EOA - PD on the frequencies of 2, 3 and 4 kHz, however, in 1 and 6 kHz, alterations occurred on the hearing sensitivity after the Cisplatin application.
![]()
Introduction
For a very long time, it has been known that various substances have toxic action and may affect a specific organ or the whole body. Owing to its sensitivity and function, outer hair cells are the first inner ear structure to be damaged by external agents. Studies revealed that innumerous substances are recognized as potentially toxic, and most of them are harmful to the cochlea whereas fewer are to the vestibule. Ototoxicity is defined as partial or total loss of vestibular/cochlear function as a result of drug use, generally with therapeutic purposes or by exposure to toxic substances or degrees of severity, depending on individual predisposition, dose, duration of treatment, administration route, age, family tendency and/or occasional previous damage to the inner ear1-12.
The conduction of a generic study in patients treated with ototoxic drugs proved to be too vast, owing to the great variety in the same class of medications. We decided, therefore, to study some of the side effects caused in users of the chemotherapy drug cisplatin using otoacoustic emissions.
Cisplatin (Cis-dichlorodiammineplatinum) is a complex heavy metal with one central atom of platinum, circled by two atoms of chlorine and two molecules of ammonia in the cis position. It is presented in the formulation of white lyophilized powder, molecular formula of PtCl2H6N2, soluble in water or saline concentration at 1mg/ml and in dymethylformamide in the concentration of 24mg/ml. (Note: needles or intravenous sets containing aluminum that can get in contact with cisplatin should not be used in the presentation or administration, since they react to form a precipitate and loss of potency).
There are innumerous techniques that can be used to monitor hearing function during the administration of ototoxic drugs. Otoacoustic emissions, as representatives of the operating conditions of the outer hair cells, can detect the initial signs of cochlear damage and, therefore, act to monitor and prevent damage caused by ototoxic agents12, 13. They are recent clinical application tests and have taken significant importance in the identification of cochlear abnormalities, especially because they form an objective exam, which is non-invasive and easy to apply. Even without defining the auditory threshold and not replacing pure tone audiometry, immitanciometry, and brainstem evoked audiometry, it provides data that no other method can14.
Evoked otoacoustic emissions - distortion product - are defined as the acoustic energy measured in the auditory canal originated by the non-linear interaction of two simultaneous pure tones applied in the cochlea15. The two pure tones are presented with different frequencies (F1 and F2), which are called the primary frequencies. In humans, the widest DPOAE occurs in the frequency equivalent to 2F1-F2 in which F1>F2.
The purpose of the present study was to conduct monitoring, using evoked otoacoustic emissions before and after use of cisplatin, of the operating conditions of outer hair cells, trying to detect signs of cochlear abnormalities after the use of the medication, enabling reassessment of dosage and prevention of future damage, preventing major hearing impairments.
Material AND METHOD
The present study was conducted by Clínica de Otorrinolaringologia Blumenau Ltda. - CLIOB, Division of Oncology, Policlínica Lindolf Bell and Centro Onco-Hematológico do Hospital Santa Catarina, between February and August 2002. Eleven subjects participated in the study and they had cancer with or without hearing complaints, both genders and ages ranging from 29 to 73 years.
We used the selection criteria that included patients starting chemotherapy protocol and using cisplatin. We collected patients' history to learn about the audiological status before treatment. All patients signed an informed consent term after they had been informed about the performance of the tests and the inclusion of their data in the study, as communicated to CRM-SC.
Evoked otoacoustic emission tests (distortion product) were conducted during the hydration period (before the application of the drug) in all patients, immediately after the application in some and within an hour interval in the others. The drug use protocol required four applications, separated by 21-day intervals, except in patients who had hematological affections. We used the device Madsen Cappela, coupled to a Toshiba Satellite Notebook.
The exams were conducted in the institution in which the patient was being submitted to chemotherapy. Not always the environment was acoustically protected; we tried to find a very silent area, considering that the greater background noise is attenuated, the better are the conditions to measures OAE. We were very careful when placing the probe in the external auditory canal so as not to have floor noise contamination or production of artifacts. Patients were tested bilaterally and we assessed the frequencies of 1, 2, 3, 4 and 6kHzm, with 65dB sound stimuli intensities.
Results
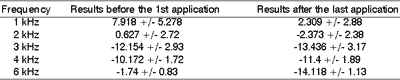
Upon comparing the otoacoustic emissions pre and post-application of cisplatin, we noticed that in frequencies 2, 3 and 4kHz there were fewer differences in response. However, in the extreme frequencies of 1 and 6kHz, there were significant changes in hearing sensitivity after the use of the chemotherapy agent.
We tested 11 patients, 6 during four cycles of cisplatin application (3 with 21-day intervals and 3 in weekly sessions), 4 conducted 3 cycles and 1 died right after the first session.
Out of the total of tested patients, 5 reported tinnitus noticed after the use of the 2nd medication. According to the literature, ototoxicity of cisplatin is primarily manifested through tinnitus or hearing loss, present in up to 31% of the patients, being the effects cumulative and irreversible12.
In the exam conducted before cisplatin application, 9 patients presented abnormal responses in DPOAE. The mean age was 54 ±17, comprising an adult population that was close to elderly life.
The performance of the test immediately after or 1 hour after application of the chemotherapy agent was made in order to observe the occurrence of a maximum peak of ototoxicity, which was not confirmed in the present study.Table 1. Exam conducted before the first application of cisplatin and after the last application: mean variations of DPOAE responses.
Discussion
Ototoxic drugs can potentially affect the cochlear mechanics, impairing hearing16. Otoacoustic emissions are very useful tests to map the function of outer hair cells in Corti's organ, enabling identification of the site in which they are damaged. It is very useful a test to monitor the risk status for hearing14.
Many authors have studied ototoxicity of cisplatin in past years; the first reports were made on 1972. Its incidence varies in the literature and it is related to dose and administration route17. Recently, we conducted a study about ototoxicity of cisplatin with a group of children and confirmed that the frequencies of 6 to 8kHz were the most affected ones, disagreeing with the data of our study (which, in turn, had the age of studied patients as an aggravating factor), except for the fact that the present study observed greater compromise of frequencies 1 and 6kHz. Other researchers, in studies conducted with Amykacin, reported the most affected frequencies were also 6 and 8kHz, which shows agreement among the data reported in the literature18.
A factor that was considered significant was the index of tinnitus. Some authors12 referred that tinnitus was a common complaint in cases of ototoxicity and described impairment of 31% of the patients. In the present study, we observed that 46% of the tested patients reported tinnitus after the second application of cisplatin, remaining so up to the end of treatment, with few intensity and frequency abnormalities.
Conclusion
After the analysis of results, we noticed that treatments conducted with cisplatin could cause abnormalities in the function of the outer hair cells. There was decrease in response, especially in extreme frequencies of 1 and 6kHz, which led to the conclusion that effective monitoring with DPOAE is essential for early detection of ototoxicity, plus the considerations about dose adjustment (without compromising the chemotherapy regimen), but with fewer effects over the hearing organ.
ACKNOWLEDGMENT
We would like to thank the subjects who agreed to participate despite the rough times they were going through, and the nursing staffs of the institutions who have been extremely helpful for the conduction of the study.
REFERENCES
1. Ayala-Martinez L. et al. Correlaciones morfofuncionales de la acion protectora del coenzima A en la ototoxia por cisplatino (II). Acta Otorrinolaringol Esp 1997;48(4):265-7.
2. Cevette MJ et al. Cisplatin ototoxicity, increased DPOEA amplitudes, and magnesium deficiency. Distortion product otoacustic emission. J Am Acad Audiol 2000;11(6):323-9.
3. Kaltenbach JA et al. Comparison of five agents in protecting the cochlea against the ototoxiceffects of cisplatin in the hamster. Otolarygol-Head-Neck-Surg 1997;117(5):493-500.
4. Laurell G et al. Blood-perilymph barrier and ototoxicity: an in vivo study in the rat. Acta Otolaryngol 2000;120(7):796-803.
5. Li L. Experimental study on cisplatinum ototoxicity. Zhonghua er bi yan hou ke za ahi 1990;25(4):205-7, 254.
6. Lou WH et al. Effect of sodium thiosulfate on cisplatin-induced ototoxicity. Zhonghua er bi yan hou ke za ahi 1990;25(4):208-10, 255.
7. Macdonald MR et al. Ototoxicity of carboplatin: comparing animal and clinical models at the Hospital for Sick Children. Otolaryngol 1994;23(3):151-9.
8. Moroso MJ & Blair RL. A review of cis-platinum ototoxicity. J Otolaryngol 1983;12(6):365-9.
9. Ozturan O et al. Monitoring of cisplatin ototoxicity by distortion-product otoacustic emissions. Auris-Nasus-Larinx 1996;23147-51.
10. Schaefer SD et al. Ototoxicity of low and moderate-dose cisplatin. Cancer 1985;56(8):1934-9.
11. Schroder M et al. Cisplatin ototoxicity. A Clinical study. Larynglo Rhinol Otol (Stuttg) 1986;65(2):86-9.
12. Silva MLG, Munhoz MSL, Ganança MM & Caovilla HH. Ototoxicoses. In: Quadros clínicos mais comuns. Vol. III. Cap. 14. Série Otoneurológica. São Paulo: Ateneu; 2000. p. 119-130.
13. Lopes Filho O & Carlos RC. Produto de distorção das emissões otoacústicas. Rev Bras de Otorrinolaringol 1996;5:224-237.
14. Munhoz MSL, Caovilla HH, Silva MLG & Ganança MM. Otoemissões acústicas. In: Audiologia Clínica. Vol. II. Cap. 9. Série Otoneurológica. São Paulo: Ateneu; 2000. p.121-148.
15. Kemp DT. Stimulates acoustic emissions from within the human auditory system. J Acoust Soc Am 1978;64:1386-91.
16. Oliveira JAA, Canedo DM & Rossato M. Otoproteção das células ciliadas auditivas contra a ototoxicidade da amicacina. Rev Bras de Otorrinolaringol 2002;68:7-14.
17. Borges GC, Borges RHM, Baraúna GN & Lopes Filho O. Ototoxicidade causada pela cisplatina em crianças - Estudo retrospectivo. Rev Bras de Otorrinolaringol 2001;67:292-296.
18. Valejjo JC et al. Detecção precoce de ototoxicidade usando emissões otoacústicas produtivas de distorção. Rev Bras de Otorrinolaringol 2001;67:845-851.
19. Boheim K & Bichler E. Cisplatin-induced ototoxicity: audiometric findings and experimental cochlear pathology. Arch-Otorhinolaryngol 1985;242(1):1-6.
20. Lopes Filho O, Carlos RC. Emissões otoacústicas. In: Tratado de Fonoaudiologia. Cap. X. São Paulo: Rocca;1996. p.221-237.
21. Martinez-Martinez MJ et al. Correlaciones morfofuncionales de la acción protectora del ácido pantotenico en la ototoxia por cisplatino (I). Acta Otorrinolaringol Esp 1997;48(4):261-4.
22. Smoorenburg GF et al. Protection and spontaneous recovery from cisplatin-induced hearing loss. Ann-N.Y-Acad-Sci 1999;28:884192-210.
23. Standnicki SW et al. Cis-dichlorodiammineplatinum (II) (NSC-119875): hearing loss and other toxic effects in rhesus monkeys. Cancer chemother 1975; Rep. 59:467-80.
24. Strauss M et al. Cis-platinum ototoxicity: clinical experience and temporal bone histopathology. Laryngoscope 1983;93:1554-9.
25. Taudy M et al. Carboplatin and cisplatin ototoxicity in guinea pigs. Audiology 1992;31(5):293-9.
26. Wright CG & Schaefer SD. Inner ear histopathology in patients treated with cis-platinum. Laryngoscope 1982; 92:1408-13.
1 Otorhinolaryngologist, CLIOB Ltda.
2 Oncologist (Sector of Oncology, Policlínica Lindolf Bell and Centro Onco-Hematológico do Hospital Santa Catarina)
3 Speech Therapist- CLIOB Ltda.
Address correspondence to: CLIOB Ltda. - Rua Prefeito Frederico Busch Jr., 255 sala 407 (CEP) Bairro Garcia Blumenau SC 89020-400
Tel/Fax (55 47) 322-0302 - E-mail ruysdael@cliob.med.br
Article submitted on December 23, 2002. Article accepted on March 27, 2003.