Year: 2008 Vol. 74 Ed. 6 - (17º)
Artigo de Revisão
Pages: 926 to 932
Steroid injection in chronic inflammatory vocal fold disorders, literature review
Author(s): Andrea Maria Campagnolo1, Domingos Hiroshi Tsuji2, Luís Ubirajara Sennes3, Rui Imamura4
Keywords: vocal fold scarring, steroids, steroid injection, inflammatory lesions of vocal fold, laryngeal disease.
Abstract:
Steroids are potent inhibitors of inflammation and wound repair. Local administration of steroids directly into the larynx has been reported in many laryngeal diseases. Aim: The purpose of this study is to review related literature about the use of steroid injection in patients with benign, inflammatory and chronic vocal disease. Methodology: We performed an electronic survey in Medline database and selected clinical trials regarding steroid use in benign laryngeal diseases. Results: Steroids are indicated in these situations: 1) acute inflammatory diseases, mainly when edema compromises the airways; 2) auto- immune disease with laryngeal involvement; 3) laryngeal stenosis; 4) benign lesions of the vocal folds, e.g., nodules, polyps and Reinke's edema, to reduce the inflammatory reactions before phonosurgery or in an attempt to avoid surgery; 5) In phonosurgery, aiming to reduce scarring. In this case, it could be used as a preventive measure in vocal fold scarring, or for scar treatment. Conclusion: Steroids may be considered an important therapeutic option in the management of many diseases, specially the inflammatory ones, associated with vocal changes.
![]()
INTRODUCTION
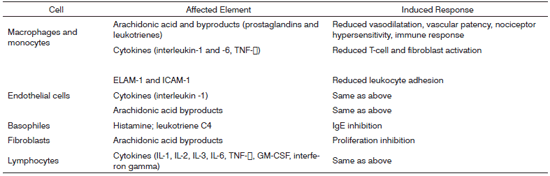
Steroids are molecules synthesized by our bodies to regulate a vast number of physiological, immune, and metabolic processes. Steroids are broadly used due to their anti-inflammatory and immunomodulation properties. They have a unique, essentially genomic (transcriptional) mode of action characterized by the activation (transactivation) or inhibition (transrepression) of numerous target genes. These molecules act upon a number of cells, involving innate (macrophages, granulocytes, mast cells) and adaptive (lymphocytes) immune responses, apart from other cells (fibroblasts, epithelial and endothelial cells). Steroid anti-inflammatory effectiveness is related to the inhibition of a number of cytokines, enzymes, and inflammation mediators and to the induction of cytokines and anti-inflammatory molecules such as lipocortin, responsible for inhibiting the release of vasoactive substances and chemotactic agents. Lipolytic and proteolytic enzymes are also reduced through lysosomal stabilization, as well as the overflow of leukocytes to sites of injury. Lymphocyte counts and degree of fibrosis are modified. All such steps will clearly affect the elements and stages of inflammatory response.1,2 (Figure 1)
Figure 1. Sites and modes of action of glucocorticoids in inflammatory and immune responses.*
* Adapted from reference 2..
Steroids inhibit multiple sites of the immune system. They interfere with both humoral and cell immune response. Research findings suggest their effect in immune disease is more related to blocking inflammatory response than inhibiting immune response.2
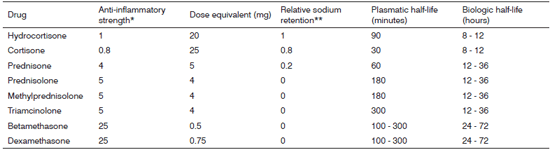
Synthetic steroidal drugs differ from each other in how strong their glucocorticoid is, and are thus dosed to provide for equal potency. They have different levels of mineralocorticoid activity and are categorized as a function of effect duration. (Figure 2)
Figure 2. Glucocorticoid equivalence for systemic administration.
* Glucocorticoid effect
** Mineralocorticoid effect
Adapted from reference 3.
The glucocorticoid equivalence table considers systemic drug administration (intramuscular, intravenous, or subcutaneous). It is important to stress the difference between plasmatic and biologic half-life. The first refers to blood hormone levels, while the latter to the time for which the drug will be available in tissue; glucocorticoid tissue presence will determine the duration of its therapeutic effect. Such duration varies depending on the salt used in the formulation of the glucocorticoid. When prepared with salts that form freely soluble ester (mono and disodium phosphate, sodium succinate), glucocorticoids are promptly taken in. On the other hand, if the steroid is prepared with other salts that form less soluble byproducts (acetate, dipropionate, acetonide, diacetate, and hexacetonide) uptake is slower.3
Glucocorticoids are the most effective anti-inflammatory agent available, as they offer symptom alleviation in a series of clinical manifestations. They, however, pose a potential risk for adverse side effects in a number of organs depending on dosage and treatment duration. When used for short periods of time (up to two weeks), even at high dosages, the probability of adverse side effects occurring is low. In prolonged therapy severe side effects set in, thus reducing their effectiveness in addressing chronic diseases.2
Local steroid injections have been used as a means of rationalizing the use of this medication. This mode of administration allows for high local drug concentration with lower risk of systemic adverse side effect.
Steroids are mostly indicated to treat acute inflammatory diseases involving the larynx, mainly in situations where edema may compromise the airways. Some studies have shown steroids being used in various chronic diseases that introduce laryngeal lesions and voice alteration.
This study aims to perform a literature review on the use of steroid injections on the vocal folds of patients with benign, inflammatory, or chronic laryngeal disease.
MATERIALS AND METHOD
A non-systematic search was performed on the MEDLINE database (from January of 1960 to November of 2006) to find medical papers reporting on the use of steroids in benign vocal fold disease.
The search for papers on Medline was done using keywords corticosteroids, steroid injection, vocal folds and laryngeal alone and in combination.
Other papers were also included besides the ones obtained from the above mentioned search.
LITERATURE REVIEW
The use of steroid on managing laryngeal disease is well established, particularly in handling conditions such as epiglottitis, croup, and laryngeal edema.4-6
Steroids are potent inhibitors of inflammation and healing.
The most common administration modes are oral, intramuscular, and intravenous. Local steroid administration in the larynx has been reported for various laryngeal diseases.
Krespi7 reported on the use of steroid injections in the lesions of six patients with laryngeal sarcoidosis.
Patients with systemic lupus erythematosus and Wegener's granulomatosis with laryngeal involvement present, in most of the cases, improvement in symptoms with steroid injections done directly on the laryngeal lesions.8,9
Success has been achieved in the treatment of patients with laryngeal stenosis with injections of triamcinolone on the site of stenosis.10,11
Some authors have reported on the use of steroid injections on the vocal folds to treat local inflammatory benign diseases such as nodules, polyps, and Reinke's edema, in an attempt to avoid surgery.
In 1967, Yanagihara12 reported good results after injecting dexamethasone on vocal folds assisted by indirect laryngoscopy in patients with nodules. This technique is however quite inaccurate, thus hampering its reproducibility. Tateya et al.13 developed a technique using a fibroscope and a curved needle introduced orally to inject triamcinolone under local anesthesia on vocal fold nodules. Twenty-three of the 27 patients enrolled in this study had nodules associated with vocal abuse and occupational stress (20 were teachers). Endoscopic findings showed that vocal fold nodules disappeared in 17 of the 27 subjects and were reduced in size in 10 patients after injection. Maximum phonation time was of 10.9s before the procedure and moved up to 13.9s afterwards, showing a significant increase (p<0.05). Remission or improvements in dysphonia were seen in 96% of the cases. There was no recurrence of adverse side effects such as reduction on vocal fold size or muscle atrophy.
These same authors14 conducted a study using this same technique to inject triamcinolone on Reinke's space of the vocal folds of 44 patients with moderate Reinke's edema. Remission and improvements were observed in almost all patients as far as dysphonia is concerned (assessed by patients) and in vocal fold endoscopic findings. The mean maximum phonation time increased significantly from 9s to 11.4s after the procedure (p<0.01). In female patients, pitch also increased significantly from 168Hz to 181Hz (p<0.05) after steroid injection.
Surgical technique and instrumentation used to remove benign vocal fold lesions and yet preserve maximum amounts of normal adjacent tissue have improved along the years. Understanding the body-cover vocal fold model is fundamental for any surgical approach. A mucosal wave is generated as the mucosal lining slides over the vocal ligament and the vocal muscle. This cover can be divided into layers with different mechanical properties, and may be differentiated by the concentration of collagen and elastin fibers. Most benign lesions take place in the superficial layer of the lamina propria, and surgical approaches should ideally be confined to this layer. Violation of the deeper layers of the lamina propria and the vocal ligament is associated with scar tissue formation. Such scar leads to the fixation of the mucosal cover to deep tissues, thus hampering the formation of the mucosal wave and causing dysphonia. Vocal fold scars are the most common cause of post-surgery dysphonia (35%).15
Steroids have been clinically used in vocal fold surgery to reduce scar tissue formation; they may also be used preventively or therapeutically against scar tissue.
Bouchayer and Cornut16 reported on the use of hydrocortisone injections on the vocal folds at the end of microsurgery to remove benign lesions such as nodules, manly when sings of inflammation, cysts, sulcus, and bridges are present. When treating iatrogenic scars, hydrocortisone is injected on the vocal fold, an incision is made in the superior surface of the vocal fold, and a microflap is raised separating it from the vocal ligament, without however removing any type of tissue. The authors reported improvements on vocal fold malleability, glottic closure, and voice quality. Courey et al.17 have also described the use of steroids under the microflap after benign lesion removal so as to reduce scar tissue formation consequent to the microflap.
Mortensen and Woo18 injected methylprednisolone on the vocal folds of 12 patients with post-surgery iatrogenic fibrosis assisted by indirect laryngoscopy under local anesthesia. Significant improvements were observed on voice quality, as measured by the GRABS scale (p<0.01). In stroboscopic examination improvements were seen on vibration amplitude (p<0.05) and mucosal wave (p<0.05). This study also looked at 18 patients with vocal folder nodules or polyps and 4 patients with sarcoidosis or granuloma. Eleven of the 18 patients with nodules or polyps had significant improvement and did not require surgery. One patient with sarcoidosis was able to avoid repeated general anesthesia procedures with steroid injection. Two patients with contact granuloma improved with steroid injections and did not require surgery. One patient required surgery and pathology test results showed vocal fold tuberculosis. Twenty-eight (82%) of the 34 patients improved their statuses. The authors reported that on-lesion steroid injections have three main indications:
1) reduce granulation tissue and promote primary healing;
2) reduce hypertrophic scar formation; and
3) reduce inflammation to avoid surgery.
The use of steroid injection to prevent vocal fold fibrosis is based only on theory, as there are no clinical or histological studies presenting consistent findings on the effect of steroids in vocal fold healing.
The only published study that histologically and functionally analyzes the effect of vocal fold steroid injection was produced by Coleman et al.19 in 1999. A microflap lateral to the vocal folds of 15 dogs was raised and triamcinolone was injected on one of the vocal folds; the other was left for control purposes. After 2, 4 and 6 weeks respectively groups of five dogs were slaughtered and vocal fold histological studies were done to analyze inflammatory infiltrate and neovascularization. Paired tests showed increased presence of inflammatory infiltrate around the microflap of vocal folds treated with steroids for 2, 4 and 6 weeks (p<0.2). Neovascularization in vocal folds treated with steroids was reduced in 2 weeks (p<0.005), but greater in 4 and 6 weeks (p<0.005). A time-dependent normal distribution curve was produced based on histological data to better characterize the effect of steroids upon the healing process. Steroid-treated tissues had responses delayed by 12 days for inflammatory infiltrate and 21 for vasculogenesis. Videostroboscopy was done preoperatively and the day the dogs were slaughtered to allow for a functional qualitative and quantitative analysis. No significant differences were found in appearance, amplitude, mucosal wave, or malleability between the two vocal folds. In this study, although the healing process was delayed by steroids, the parameters assessed during videostroboscopy did not show differences between treated and control vocal folds after 2, 4 and 6 weeks.
Scars are generally related to fibrous tissue. Studies have shown that increases in collagen - especially type 1 collagen - promote fibrosis, and that collagen content has direct impact on scar strength.20 In the case of vocal fold fibrosis, dense collagen deposits have also been found in the injured lamina propria of animal model vocal folds21-23. However, recent research suggests that other components of the extracellular matrix such as hyaluronic acid, fibronectin, and decorin may also affect fibrous tissue. Although collagen is the main molecule, it is not the only one to determine tissue viscoelastic properties.24
A study on the role of monocytes/macrophages in healing, subcutaneous deposit hydrocortisone at 0.6mg/g of bodily weight was given to pigs to induce long term monocytopenia. Macrophage level in the wound was reduced to 1/3 in relation to control subjects. Fibroblasts would normally appear within three days, but were not seen before 5 days and their proliferation was reduced on the healing site.25
Injections of deposit steroids such as triamcinolone have been used in hypertrophic scars and cheloid. Carroll et al.26 showed in vitro that collagen and cell proliferation reduction promoted by triamcinolone is mediated by alterations in cytokine secretion.
A study is currently being conducted in animal models at our service to histologically assess whether steroids can be used in the formation stages of the extracellular matrix components as an alternative to reduce vocal fold mucosal stiffness introduced by postoperative healing. Twelve rabbits were used in this study. All subjects got dexamethasone injections through a lateral microflap raised in one of their vocal folds, while the other vocal fold was left with the microflap untreated for control purposes. Histology tests were done to assess inflammatory infiltrate and amount of collagen after three and seven days, during acute inflammatory response. In terms of inflammatory infiltrate, there was no difference in the number of cells around the microflap. Amounts of collagen, however, were significantly lower in the steroid-treated vocal fold after three days, and this difference was sustained after seven days. 27
DISCUSSION
Steroids are broadly employed in various laryngeal diseases, mainly because of their powerful anti-inflammatory properties. Alongside benefits are, however, various potential adverse side effects that may involve a series of tissues depending on dosage and treatment duration. Any agent can be administered in systemic acute conditions, as undesired side effects are not observed.
When the therapeutic goal involves reaching higher concentrations in one single target - e.g.: larynx - local steroid injection is effective and introduces fewer systemic side effects, as long as dosage is strictly observed and treatment is short term. Longer half-life steroids such as triamcinolone, methylprednisolone acetate, and dexamethasone are preferably used in local injections. Triamcinolone is broadly used in joint injections in the orthopedic practice28,29, with confirmed effectiveness and safety. Bouchayer and Cornut16 used vocal fold hydrocortisone injections to reduce present signs of inflammation, but probably due to shorter medication half-life the impact on healing was limited. In theory, longer half-life steroids do a better job of preventing fibrous tissue formation.
The main adverse effect of this treatment mode is muscle and gland atrophy. None of the papers referred to in this study reported such event. However, Mortensen and Woo18 suggest patients with fibrosis should have intervals of 6 to 12 weeks between injections and those suffering from inflammatory lesions should either have one single injection or more in longer intervals.
Many autoimmune diseases such as systemic lupus erythematosus, Wegener's granulomatosis, and sarcoidosis may present laryngeal lesions that cause dysphonia and respiratory involvement. Most symptoms are resolved with steroid therapy, usually employed for longer periods of time. Immunosuppressant drugs and chemotherapy medication (azathioprine, cyclophosphamide, and methotrexate) are also associated to reduce or stop steroid administration. However, patients with severe laryngeal involvement have improved when taking local steroid injections, possibly an alternative with fewer adverse side effects.
Healing modulation is the biggest factor in the success of stenosis surgical treatments. Steroids were tested with this purpose, but their use remains controversial30. On the one hand, they act on collagen formation and degradation, reducing granulation tissue formation, as observed by Gnanapragasam10 and Rosen and Vered11. On the other hand, they interfere with the migration of epithelial cells and on the healing of developing ulcers, which may get infected and increase the chances of restenosis. Local steroid injection may also produce cartilage resorption.31 Mitomycin-C has shown more promising results than steroids to treat laryngeal stenosis.32
Acquired benign laryngeal lesions affecting the vocal folds are the consequence of inflammatory processes that stem basically from three factors: vocal fold abuse or poor use of the voice (trauma), irritative processes (smoking, pharyngeal-laryngeal reflux, pollution etc), and individual predisposing factors.33
Vocal fold nodule disease is characterized by the distension of capillaries and venules, mild perivascular hemorrhage, tissue fibrin migration, diffuse edema, and fibrosis. Polyps, Reinke's edema, and contact granuloma also have inflammatory traits. Thus, local steroid injections are a rational and particularly effective means to treat these lesions in their early stages, before fibrous tissue has developed.13,33
Vocal fold fibrosis is caused by injury and inflammation. Inadequate surgical technique can easily introduce fibrous scars on the vocal folds. These scars disorganize the vocal fold mucosal lamina propria and change the biomechanical properties of the vocal folds, as mucosal viscoelastic strength is increased. This, in turn, reduces vocal fold vibration and causes dysphonia, a condition difficult to be treated.24
No treatment method can yet be considered effective in resolving vocal fold fibrosis.24 Steroid injections are among the most broadly employed treatments and have been associated with reduced rigidity, improved glottal closure, and good resulting voice quality, as observed by Bouchayer and Cornut16 and Mortensen and Woo18. Nonetheless, some degree of fibrosis always remains in place. Many other substances have been attempted, such as bovine collagen34, autologous collagen35, and autologous fat36 to enhance vocal fold flexibility. However, none of these materials is able to restore the changes introduced in the lamina propria by fibrosis and repair viscoelasticity to levels identical to those found in normal vocal folds.
As the treatment options for vocal fold fibrosis are limited, studies have been conducted to prevent fibrosis formation. Many surgeons are using steroid injections on surgical wounds to enhance injury healing. Steroids affect collagen synthesis and maturation, change wound tensile strength, inhibit fibroblast function, and suppress defense cell antibacterial and phagocytic properties, resulting in modified patterns and delayed wound healing37. The effect is more visible within the first four days of treatment. Aside from inhibiting normal infection resistance, after four days steroids have almost imperceptible effect38. According to Branski et al.39, the acute stage of the healing process is critical. Therapeutic interventions introduced at this time may reduce vocal fold scar stiffness, as inflammatory cells and fibroblasts start synthesizing extracellular matrix components two or three days after injury. If fibrosis prevention is the goal, the right time to use long half-life steroids is at the onset of the inflammatory process, i.e., at the end of surgery.
The alterations introduced by steroids in collagen synthesis were reported by Campagnolo e Tsuji27, but they are associated only with the acute inflammatory stages. Coleman et al.19 followed their patients for 6 weeks and found no difference in vocal fold vibration under functional stroboscopic assessment. Histological examination did not consider matrix components (collagen, elastin etc) and consequently their association with vocal fold mucosa vibration; they looked only at inflammatory infiltrate and neovascularization. In spite of the proven effect of steroids in healing, more studies are required mainly to assess whether collagen changes persist in the long run.
Reports on the use of mitomycin-C have not shown benefit in preventing fibrosis.40 Encouraging results were recently reported from liver cell growth factor injections, as a potent anti-fibrotic agent.41 Ideal prevention and treatment modes for vocal fold fibrosis are yet to be established.
CONCLUSION
Systemic steroids are recommended to treat acute laryngeal inflammation. In chronic inflammatory disease, steroids may be used systemically or injected locally on the lesion in more severe cases.
Local steroid injections are advantageous as they allow higher drug concentrations on the target with reduced potential for adverse systemic side effects. They may be indicated to 1) reduce edema, benign mucosal lesion, and granuloma size before surgery or as an attempt to avoid it, and 2) minimize post-surgery iatrogenic scar. Although many authors reported on local steroid injection at the end of surgery to prevent scar formation, such application remains controversial.
Steroids may be considered as an important treatment option for various diseases, mainly inflammatory ones that adversely affect the vocal folds.
REFERENCES
1. Sibilia J. Corticosteroids and inflammation. Rev Prat 2003;53(5):495-501.
2. Wannmacher L, Ferreira MBC. Antiinflamatórios esteróides. In Fuchs D, Wannmacher L, editores. Farmacologia Clínica. 2nd ed. Rio de Janeiro: Guanabara Koogan; 1998. p. 194-202.
3. Louzada P, Sarti W. Corticosteróides. In Campos CAH, Costa HOO, editores. Tratado de Otorrinolaringologia. São Paulo: Roca; 2002. p.243-9.
4. Rosekrans JA. Viral croup: current diagnosis and treatment. Mayo Clin Proc 1998;73:1102-6.
5. Kairys SW, Olmstead EM, O' Connor GT. Steroid treatment of laryngotracheitis: a meta-analysis of the evidence from randomized trials. Pediatrics 1989;83:683-93.
6. Ossoff RH, Wolff AP, Ballenger JJ. Acute epiglottitis I adults: experience with fifteen cases. Laryngoscope 1980;90:1155-61.
7. Krespi YP, Mitrani M, Husain S, Meltzer CJ. Treatment of laryngeal sarcoidosis with intralesional steroid injection. Ann Otol Rhinol Laryngol 1987;96:713-5.
8. Teitel AD, MacKenzie CR, Stern R, Paget SA. Laryngeal involvement in systemic lupus erythematosus. Semin Arthritis Rheum 1992;22:203-14.
9. Gulatti SP, Sachdeva OP, Sachdeva A, Singh U. Wegener's granulomatosis: a care with laryngeal involvement. Indian J Chest Dis Allied Sci 1997;39:125-8.
10. Gnanapragasam A. Intralesional steroids in conservative management of subglottic stenosis of the larynx. Int Surg 1979;64(3):63-7.
11. Rosen G, Vered IY. Triamcinolone acetonide injection for laryngeal stenosis. J Laryngol Otol 1975;89(10):1043-6.
12. Yanagihara n, Azuma F, Koike Y, Honjo I, Imanishi Y. Intracordal injection of dexamethasone. Practica Otorhinolaryngol 1964;57:496-500.
13. Tateya I, Omori K, Hirano S, Kaneko K, Ito J. Steroid injection to vocal nodules using fiberoptic laryngeal surgery under topical anesthesia. Eur Arch Otorhinolaryngol 2004;261(9):489-92.
14. Tateya I, Omori K, Hirano S, Kaneko K, Ito J. Steroid injection for Reinke's edema using fiberoptic laryngeal surgery. Acta Otolaryngol 2003;123(3):417-20.
15. Woo P, Casper J, Colton R, Brewer D. Diagnosis and treatment of persistent dysphonia after laryngeal surgery: a retrospective analysis of 62 patients. Laryngoscope 1994;104:1084-91.
16. Bouchayer M, Cornut G. Microsurgery for benign lesions of the vocal fold. Ear Nose Throat J 1998;67:446-66.
17. Courey MS, Gardner GM, Stone RE, Ossoff RH. Endoscopic vocal fold microflap: a three-year experience. Ann Otol Rhinol Laryngol 1995;104:267-73.
18. Mortensen M, Woo P. Office steroid injections of the larynx. Laryngoscope 2006;116(10):1735-9.
19. Coleman JR, Smith S, Reinisch L, Billante CR, Ossof J, Deriso W, Garret CG. Histomorphometric and laryngeal videostroboscopic analysis of the effects of corticosteroids on microflap healing in the dog larynx. Ann Otol Rhinol Laryngol 1999;108:119-27.
20. Ehrlich HP. Collagen considerations in scarring and regenerative repair. In Garg HG, Longaker MT eds. Scarless wound healing. New York, NY: Marcel Dekker Inc.; 2000. p. 99-113.
21. Hirano M, Phonosurgery. Basic and clinical investigation. Otologia (Fukuoka) 1975;21(suppl 1):299-303.
22. Rogerson AR, Clark KF, Bandi SR, Bane B. Voice and healing after vocal fold epithelium removal by CO2 laser vs. microlaryngeal stripping. Otolaryngol Head Neck Surg 1996;115:352-9.
23. Blakeslee DB, Banks RE, Eusterman V, Brooks D. Analysis of vocal fold function in the miniswine model. J Invest Surg 1995;8:409-24.
24. Hirano S. Current treatment of vocal fold scarring. Curr Opin Otolaryngol Head Neck Surg 2005;13(3):143-7.
25. Leibovich SJ. The role of the macrophage in wound repair. A study with hidrocortisone and antimacrophage serum. Am J Pathol, 1975;78(1):71-100.
26. Carroll LA, Hanasono MM, Mikulec AA, Kita M, Koch RJ. Triamcinolone stimulates bFGF production na Inhibits TGF-[beta]1 production by human dermal fibroblasts. Dermatol Surg, 2002;28(8):704-9.
27. Campagnolo AM, Tsuji DH. Estudo histológico da ação do corticosteróide intralesional no processo agudo de cicatrização da lâmina própria das pregas vocais de coelhos. Comunicação pessoal. Tese de doutorado ainda não publicada. USP, São Paulo, SP.
28. Boehnke M, Behrend R, Dietz G, Kuster RM. Intraarticular hip treatment with triamcinolonehexacetonide in juvenile chronic arthritis. Acta Univ Carol 1994;40:123-6.
29. Caldwell JR. Intra-articular corticosteroids. Guide to selection and indications for use. Drugs 1996;52:507-14.
30. Ingrams DR, Sukin SW, Ashton P, Valtonen HJ, Pankratov MM, Shampshay SM. Does slow-release 5-fluouracil and triancinolone reduce subglottic stenosis? Otolaryngol Head Neck Surg 1998;118(2):174-7.
31. Estenose laríngea. Disponível em http://www.google.com/search?q=cache:Rdu-pC5HCpIJ:www.hcnet.usp.br/otorrino/sem62.doc+estenose+lar%C3%ADngea&hl=pt-BR&gl=br&ct=clnk&cd=1. Acessado em 18 de dezembro 2006.
32. Sennes LU, Tsuji RK, Imamura R, Tsuji DH. O uso da mitomicina-c no tratamento endoscópico de estenoses supraglóticas. Arq Int Otorrinolaringol 2003;7(1):224-7.
33. Crespo NA, Maunsell R. In Campos CAH, Costa HOO, editores. Tratado de Otorrinolaringologia. São Paulo: Roca; 2002. p. 421-9.
34. Ford CN, Bless DM. Collagen injection in the scarred vocal fold. J Voice 1987;1:116-8.
35. Ford CN, Staskowski PA, Bless DM. Autologous collagen vocal fold injection: a preliminary clinical study. Laryngoscope 1995;105:944-8.
36. Brandenburg JH, Kirkham W, Koschkee D. Vocal cord augmentation with autogenous fat. Laryngoscope 1992;102:495-500.
37. Kloth LC, McCulloch JM. The inflammatory response to wounding. In McCulloch JC, Kloth LC, Feedar JA eds. Wound healing alternatives in management. Philadelphia: CPR F. A. Company; 1998. p. 3-15.
38. Piccinato CE, Netto JC, Cherri J. Cicatrização. In Campos CAH, Costa HOO, editores. Tratado de Otorrinolaringologia. São Paulo: Roca; 2002. p. 189-200.
39. Branski RC, Verdolini K, Rosen CA, Hebda PA. Acute vocal fold wound healing in a rabbit model. Ann Otol Rhinol Laryngol 2005;114:19-24.
40. Garret CG, Soto J, Riddick J, Billante CR, Reinisch L. Effect of mitomycin-c on vocal fold healing in a canine model. Ann Otol Rhinol Laryngol 2001;110:25-30.
41. Hirano S, Bless DM, Rousseau BMA, Welham NMSLT, Montequin D, Chan R, Ford CN. Prevention of vocal fold scarring by topical injection of hepatocyte growth factor in a rabbit model. Laryngoscope 2004;114(3):548-56.
1 MD, Otorhinolaryngologist, PhD student in the Medical School at Universidade de São Paulo, professor of Otorhinolaryngology at Faculdade de Medicina de Campos.
2 Associate Professor at Hospital das Clínicas da USP, Assistant Physician at Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo.
3 Associate Professor at Hospital das Clínicas da USP, Associate Professor of the Otorhinolaryngology Program at the Medical School at Universidade de São Paulo.
4 PhD in Otorhinolaryngology at Hospital das Clínicas da USP, Assistant Physician at Hospital das Clínicas da Universidade de São Paulo.
Faculdade de Medicina da Universidade de São Paulo.
Send correspondence to: Andrea Maria Campagnolo - R. Marquês de Herval 11/402 Parque Tamandaré 28035-013 Campos RJ.
E-mail: amcampag@ig.com.br
This paper was submitted to the RBORL-SGP (Publishing Manager System) on 4 January 2007. code 3579.
The article was accepted on 20 August 2007.