Year: 2003 Vol. 69 Ed. 2 - (2º)
Artigo Original
Pages: 151 to 158
The clinical and histological behavior of rat skin submitted to topical and injected Mitomycin C
Author(s):
Prof. Dr. Fernando de Andrade Quintanilha Ribeiro[1],
Janaina de Pádua Borges[2],
Dra. Flávia Fernandes Silva Zacchi[3],
Dra. Lusiele Guaraldo[4]
Keywords: mitomycin-C, fibroblast inhibition.
Abstract:
Objective: The proven ability of Mitomycin-C of inhibiting fibroblasts in vitro, has stimulated its use in research animals and in humans to control healing. The objective of this prospective experimental study was to follow the healing process of surgical wounds in the dorsum of rats treated topically with Mitomycin-C. It also evaluates the skin response to injections of different concentrations of this drug. Material and Methods: Two distinct surgical wounds were made to the dorsum of ten adult rats, one of them received topical Mitomycin-C diluted at 0.5 mg/mL during 5 minutes and the other wound was used as a control. Three other rats were submitted to intradermal injections of Mitomycin C at different concentrations. The healing process of all three groups of rats was followed clinically and histologically after sacrificing the rats at different post-treatment periods. The degree of fibrosis was evaluated histologically by two distinct pathologists. Results: Surgical wounds treated with Mitomycin-C presented delayed healing when compared to the untreated wounds, with remission of scabs 7 days after the control wounds. Histological analysis at one month post-treatment revealed a significant reduction in fibrosis of the wounds treated with Mitomycin-C when compared to the untreated wounds. After the third month the degree of fibrosis was comparable in both wounds. As for the injected dilutions, clinical and histological necrosis was proportional to the concentration (0.5; 0.1; and 0.05 mg/ml). The only concentration at which no necrosis was observed was 0.01 mg/ml. Conclusion: Topical Mitomycin-C delays the healing of surgical wounds in rats up to the forth week following treatment, but the degree of fibrosis is comparable in both wounds after 12 weeks. Its intradermal use cased necrosis only at high concentrations. In otolaryngology this characteristic of the drug may be useful in the treatment of external ear canal stenosis, coanal atresias, nasal cicatricial stenosis, laryngeal stenosis and keloids.
![]()
Introduction
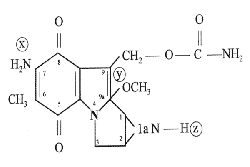
ENT Surgeries, especially cavity surgeries, commonly present cases of stenosis and synechias, both congenital and acquired, mostly iatrogenically. Despite proper surgical technique, relapse of such cases is inconveniently frequent. Many adjuvant processes in surgery have tried to mitigate such issues, as the use of drugs and beta therapy does not always provide good outcomes. A promising solution for such cases is topical use of Mitomycin C, which has been empirically used by some colleagues worldwide. This drug is an antibiotic isolated from Streptomyces caespitosus by Wakaki et al. in 1958, in the shape of violet-blue crystals, molecular weight of 334 and soluble in water and organic solvents. Its molecule has three groups known as carcinostatic: an aziridine ring, a quinone group and an octane group (Fig 1). It is commonly used as chemotherapy agent, in IV applications to treat chorionepithelioma, reticular cell sarcoma, seminoma and skin tumors including mouth, lungs, bowels, pancreas and stomach tumors. In some cases, it is used as contact anti-neoplastic therapy, mainly in bladder tumors (1,2,3). Severe side effects found included spinal aplasia with IV dose higher than 50mg and localized tissue necrosis if drug leaks to subcutaneous tissue. Several studies in literature proved the effectiveness of this drug as tumor-growth inhibitor due to its direct activity on DNA. "In vitro" studies showed its fibroblast-inhibiting ability (4) and this characteristic, after animal research studies, led to the beginning of its use in ophthalmology to control relapse of pterygium and glaucoma (7,8,9). Few ENT studies were conducted only with research animals to study healing of laryngeal-induced trauma (10,11,12,13,14), and to delay healing process of drum membrane paracentesis that could be used as alternative to surgery to insert ventilation tube (15, 16,17,18,). One study demonstrated its chance to be used topically in the endolymphatic sac to treat Ménière disease (19). In the field of general surgery, it was studied in order to inhibit intra-abdominal synechias (20). None of these studies, however, was based on detailed studies or on well-defined method.
Objective
To conduct a clinical and histological study in rats with the comparative healing process of two surgical wounds treated only with Mitomycin C. To study clinical and histological behavior of the site injected with different concentrations of the drug.
Material and Methods
The study was conducted at the Department of Technical and Experimental Surgery of the Medical School of Santa Casa, Sao Paulo, and histological studies were performed at the Department of Clinical Pathology.
Ten young adult male rats were used to study topical behavior of the drug, and to evaluate intradermal concentrations, three Wistar females aged from 180 and 210 days and weighing 335 to 400 g were placed in individual cases in the Animal Lab of the Department of Surgical Techniques.
About surgical wounds:
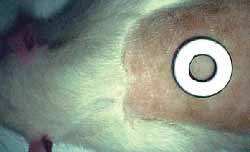
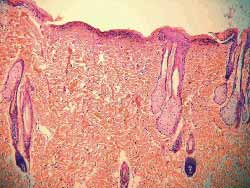
Two round similar surgical wounds were performed on the dorsum of each one of the 10 rats after shaving and under general anesthesia. Anesthetic drug used were Ketamine (Francotar® ) -10 ml with 100 mg/ml concentration at a dose of 0.5ml/rat, and Xilasine (Rompun ®) 10 ml with 20 mg/ml concentration at a dose of 0.1m/rat. The same Standard measurement device (washer) was used to make the wounds (Fig 2).The dorsum of the rat was chosen in order to prevent animals from self-inflicting damage (Fig 3). Cranial wound was labeled as A and caudal wound was labeled as B. Size was 1 cm of diameter and the distance between them was approximately 5 cm. One of the wounds received Mitomycin C topically under 0.5 mg/ml concentration for 5'. This concentration was chosen firstly because the drug would precipitate at higher concentrations, and secondly because this was the concentration typically used in the literature with no reports of local lesions of any type (9,12,13,15). First, 2 ml of the drug were dropped over gauze that covered the wound (Fig 4). Then, the wound was washed with 10 ml of free saline solution. The same procedure was used with wounds not treated with Mitomycin C. In other words, 2 ml of saline solution were applied in such wounds for 5', then they were washed with 10 ml of saline solution. Cranial and caudal wounds were randomly used for the treatment with Mitomycin C in order to prevent any differences in healing site. Rats were numbered from one to 10, both in the cages and with ink labeling system over their tails. Animals were followed up weekly for visual checking of healing by two different observers, and were sacrificed afterwards with intracardiac injection of KCL at 19.1% under general anesthetic drug. Total animals sacrificed were four rats in 4 weeks and six rats in 12 weeks, wounds were surgically removed and sent to histological test (hematoxylin-eosin). Two skilled clinical pathologists that did not know which dorsum scars or caudal scars were treated with Mitomycin C examined the scars and classified them in terms of fibrosis degree. Blood sample was collected when animals were sacrificed (ratio 1 out of 3) for blood analysis in order to check likelihood of hematopoietic changes.
About intradermal injection:
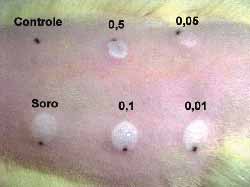
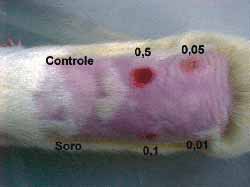
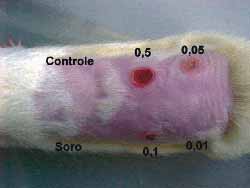
Intradermal injections were given in the dorsum of three rats after shaving and under sedation as above mentioned. Six sites were chosen. One was used as control (above to the left) and five (5) intradermal injections were injected in each one of the sites. The first one (below to the left), was injected with saline solution. The others received different concentrations as follows: 0.5 mg/ml (above, in the center); 0.1 mg/ml (below, in the center); 0.05 mg/ml (above to the right) and 0.01mg/ml.(below to the right) (Fig 5).The sites injected were assessed daily and after seven days animals were sacrificed and injected sites were subjected to histological examination.
Results
About topical use:
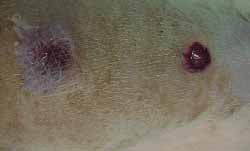
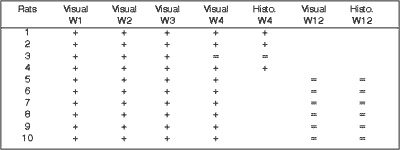
Visual observation of healing time of wounds was the same for all rats. All wounds had crust formation on the days following surgery. In wounds not treated with Mitomycin C these crusts decreased until they fell, about 2 weeks later. In the wounds treated with Mitomycin the crusts lasted for approximately 3 weeks (Fig 6). Visual observation performed by two observers reported a difference in the healing process in all rats, except for one, until the 4th week. In week 12, all scars were similar, and differentiation between treated and untreated wounds with Mitomycin C was not possible.
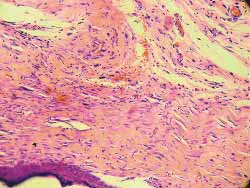
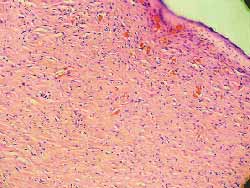
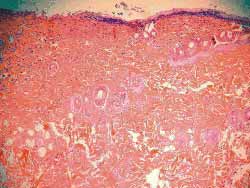
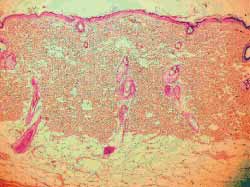
The analysis findings of the four rats sacrificed after 4 weeks were as follows: Histology of (hematoxylin-eosin) wounds treated with Mitomycin C (Fig 7) showed decreased degree of fibrosis against those not treated with this drug (Fig 8), except for one rat in which these wounds were similar. The analysis of six rats sacrificed after 12 weeks did not report any histological difference for degree of fibrosis between treated and control wounds (Figs 9, 10) (Table 1).
Blood tests of the rats carried out in the central laboratory of Santa Casa de Misericórdia de Sao Paulo were similar and within normal ranges, with no signs of medullar aplasia even in the animals sacrificed earlier.
Figure 1. Mitomycin C molecule.
Figure 2. Washer on the dorsum of the rat
Figure 3. One-cm wounds (A and B) on the dorsum of rats
Figure 4. Gauze soaked in Mitomycin C over the wound, applied for 5 minutes
Figure 5. Dorsum of a rat with the control area, the area injected with saline solution and the different concentrations
Figure 6. Observation of the rat dorsum, in which the wound not treated with mitomycin C was already closed, differently from the treated ones
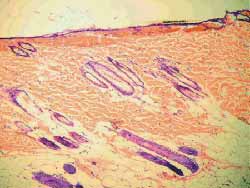
Figure 7. Less marked fibrosis on the wound treated with mitomycin C (4 weeks) (100x magnification)
Figure 8. More marked fibrosis on the wound not treated with mitomycin C (4 weeks) (100x magnification)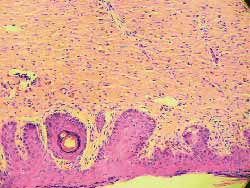
Figure 9 and 10. More marked fibrosis on the wound not treated with mitomycin C (4 weeks) (100x magnification)
Figure 11. Dorsum of the rat with the control area, injected with saline solution and the various concentrations of the drug. Note the various degrees of necrosis and the normal tissue at the concentration of 0.01 mg/ml of mitomycin C
Figure 12. (A) control, absence of abnormalities (100x magnification)
About injected concentrations:
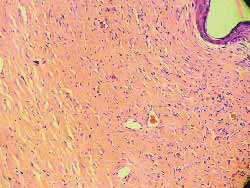
A change in color of the injected sites was reported from the second day on in three rats with 0.5, 0.1 and 0.05 mg/ml concentrations. The comparison between the sites injected with saline solution with 0.01 mg/ml concentration did not show any difference against the control site. Skin necrosis after this day on was proportionally more intense in injected sites and only with the concentration of 0.01 mg/ml; necrosis was not visually observed (Fig 11). Rats were sacrificed on the 7th day and sites were subjected to histological study (hematoxylin-eosin- 100x). The same findings (necrosis proportional to concentration) were found, with 0.01 mg/ml concentration, no tissue lesions were observed under microscope analysis, a result comparable to those of the control sites injected with saline solution.(Figs-12,13,14,15,16,17).
Discussion
Mitomycin C has been used for some time as a chemotherapy agent both intravenously and topically. It shows some in vitro fibroblast-inhibiting activity that resulted in its use mainly in ophthalmology to inhibit healing process. It has been used in pterygium and glaucoma with some prospective good results as relapse inhibitor. Some trial studies have been conducted in ENT field, but without any defined and consistent method.
About its topic use:
Mitomycin C at the concentration of 0.5 mg/ml does not cause any local involvement when topically used. Wound healing on the sites where Mitomycin C was applied was slower (crusts dropped after 21 days) than that found in untreated wounds (crusts dropped after 14 days). Healing was delayed in the wounds treated with Mitomycin C until the first month, and during this time period, weekly follow-up carried out separately by two observers did not detect which wounds were treated with the drug. Still in the 4th week, it was possible to find healing differences in all rats except for one, in which observers were not able to differentiate any factors. During the 12-week observation period, no visual differences were found between the wounds treated with Mitomycin C and untreated wounds. They seemed to heal in the same way. Histological examination of the scars (4 weeks) initially showed a decrease in fibrosis in the wounds treated with Mitomycin C against control wounds. Subsequently, the fibrosis process was equal for both wounds. Therefore, there was a delay in healing, but not a final inhibition of fibrosis degree.Quadro 1. O sinal + determina quando ambos os observadores conseguiram diferenciar as feridas tratadas das não tratadas com Mitomicina C, quer na observação visual quer na histológica. O sinal = quando ambas as feridas se encontravam iguais.
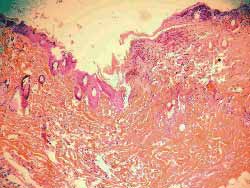
Figure 13. (B) Saline solution, superficial erosion of the recovering or already recovered epithelium (needle point) (100x magnification)
Figure 14. (C) 0.5 extensive necrosis of epidermis and dermis, sometimes even subcutaneous area, with build up of pyocytes, mild proliferation of fibrous connective tissue and destruction of skin annexes (100x magnification)
Figure 15. (D) 0.1 necrosis more superficial than in C, with exsudate of pyocytes, more mild than in C, with marked inflammatory process and maintained skin annexes. Homogenization of collagen tissue (100x magnification)
Figure 16. (E) 0.05 necrosis of dermis and epidermis more superficial than in D, scarce pyocyte exsudate and skin annexes maintained (100x magnification)
Figure 17. (F) 0.01: no abnormalities (100x magnification)
The evaluation of the histological characteristics of the scars was carried out by two third party examiners (skilled clinical pathologists) at random. Results found were the same in all cases, showing correctness in observations.
Blood tests of the animals were normal proving that toxic effect of Mitomycin C on hematopoietic tissue, if administered intravenously and at high doses, was not reported at such small dose and in topical use.
About injectable use:
Mitomycin C IV is not clinically used because it is reported that this chemotherapy application in tumors leaks to other tissues and results in local necrosis. This part of the study aimed at clarifying which concentration level would not result in necrosis.
In three rats it was found that if 0.5 mg/ml concentration used topically were to be applied IV, it would result in major local necrosis, which was also intense under the concentration of 0.1 mg/ml, and was less evident at 0.05 mg/ml. No significant findings were reported at 0.01 mg/ml in comparison to saline solution treated wounds or normal skin. The authors believe that at this concentration the drug has some fibroblast-inhibiting effect, but does not damage tissues. This characteristic may be valuable for additional injectable treatment of surgical wounds initially treated with topical Mitomycin C.
For this reason, the authors of this study believe that Mitomycin C could be used as adjuvant drug in all surgeries in which healing process delay is required such as stenosis, and synechias. We would also like to point out that after three months, it seemed to have similarity in the healing process and this would be a hindrance for this indication, therefore injectable drug could be used additionally. Thus, this drug could be used in ENT stenosis of external acoustic meatus, laryngeal stenosis, nasal synechias, choanal perforations, ostium permeability of paranasal sinuses, etc. In general surgery it could be used as keloid and general synechia and stenosis inhibitor.
Conclusions
1. Mitomycin C is not toxic for rats at 5mg/ml, if used topically in surgical wounds for 5 minutes and washed afterwards with saline solution.
2. Topical Mitomycin C at this dose delayed the healing process of surgical wounds on the dorsum of rats.
3. Histological study of Topical Mitomycin at this dose reported that it inhibited healing fibrosis process of surgical wounds on the dorsum of rats in the first 4 weeks.
4. Topical use of Mitomycin C at 0.5 mg/ml did not have any effect on healing of wounds on the dorsum of rats after 12 weeks.
5. Mitomycin C does not cause tissue necrosis if injected at 0.01 mg/ml in the skin of the dorsum of rats.
References
1. Bradner WT. Mitomycin C: a clinical update. Cancer Treat Rev 2001; Feb 27 (1):35-50
2. Crooke S T, Bradner W T. Mitomycin C: a review. Cancer Tret Rev 1976; 3:121-39
3. Bruijn E A, Sleeboom H P, van Helsdinger PJ R O, van Oosterom A T, Tjaden U R, Maes R A A. Pharmacodynamics and pharmacokinetics of intravesical Mitomycin C upon different dwelling times. Int J Cancer 1992; 51:359-64
4. Hu D, Sires BS, Tong DC, Royack GA, Oda D. Effect of brief exposure to mitomycin C on cultured human nasal mucosa fibroblasts. Ophthal Plast Reconstr Surg 2000; Mar16 (2):119-25
5. Sampaio M W, José N K, Alves M R. Efeitos do uso tópico da Mitomicina C em olhos de ratas. Arq. Bras Oftal. 1995; 58:56-9
6. Mattar D B, Alves M R, Silva M H T, José N K. Estudo da influência da aplicação subconjuntival da Mitomicina C na reparação de defeito epitelial corneano, em coelhas. Arq Bras Oftal 1995; 58:6567
7. Martins-Neto A, Paro G, Schellini SA, Moraes-Silva MRB. Mitomicina C no intra-operatório do pterígio. Arq Bras Oftalmol 1996; 59:397.
8. Paro G, Martins-Neto A, Schellini SA, Padovani CR. Nossa experiência com o colírio de Mitomicina C no pós-operatório do pterígio. Arq Bras Oftalmol 1996;59:398-1
9. Mandia C J. Trabeculectomia com Mitomicina C no tratamento de glaucomas refratários., Tese de Doutorado, Faculdade Medicina de Ribeirão Preto, 1997.
10. Ingrams DR et al,. Sinus surgery: does mitomycin-C reduce stenosis? Laryngoscope 1998; 108:883-6.
11. Spector J E, Werkhaven J A, Spector N C, Huang S, Page R N, Baranowski B, Luther M, McGehee B, Reinisch L.- Preservation of function and histologic appearance in the injured glottis with topical mitomycin C. Laryngoscope 1999;109:1125-29.
12. Garrett CG, Soto J, Riddick J, Billante CR, Reinisch L. Effect of mitomycin-C on vocal fold healing in a canine model. Ann Otol Rhinol Laryngol 2001; Jan;110(1):25-30
13. Rahbar R, Shapshay SM, Healy GB. Mitomycin: effects on laryngeal and tracheal stenosis, benefits, and complications. Ann Otol Rhinol Laryngol 2001; Jan;110(1):1-6
14. Coppit G, Perkins J, Munaretto J, Nielsen R, McKinney L, Ulnick K. The effects of mitomycin-C and stenting on airway wound healing after laryngotracheal reconstruction in a pig model. Int J Pediatr Otorhinolaryngol 2000; Jun 30;53(2):125-35
15. Jassir D, Buchman CA, Gomez-Marin O. Safety and efficacy of topical mitomycin C in myringotomy patency. Otolaryngol Head Neck Surg 2001; Apr 124(4):368-73
16. Estrem S A, Batra P S. Preventing myringotomy closure with topical Mitomycin C in rats. Otolaryngology-Head and Neck Surgery 1999;120(6):794 98
17. Estrem S A, van LeeuwenR N. Use of Mitomycin C for maintaining myringotomy patency. Otolaryngology-Head and Neck Surgery 2000 ; 122(1):8-10
18. O'Reilly RC, Goldman SA, Widner SA, Cass SP. Creating a stable tympanic membrane perforation using mitomycin C. Otolaryngol Head Neck Surg 2001; Jan;124(1):40-5
19. Yazawa Y, Suzuki M, Kitano H, Kitajima K. Intraoperative mitomycin-C in endolymphatic sac surgery for Ménière´s disease: a pilot study. ORL J Otorhinolaryngol Relat Spec 1999; 61: 188-94.
20. Cubukcu A, Alponat A, Gonullu NN, Ozkan S, Ercin C. An experimental study evaluating the effect of Mitomycin C on the prevention of postoperative intrabdominal adhesions. J Surg Res 2001; Apr;96(2):163-6
1 Chefe de Clínica Adjunto, Dep. O.R.L., Faculdade de Ciências Médicas da Santa Casa de São Paulo.
2 Pós-graduanda do Dep. De Farmacologiada da USP e farmacêutica da Técnica Cirúrgica da Faculdade de Ciências Médicas da Santa Casa de São Paulo.
3 Residente do Departamento de Anatomia-Patológica da Faculdade de Ciências Médicas da Santa Casa de São Paulo.
4 Professora Adjunto do Departamento de Ciências Fisiológicas da Faculdade de Ciências Médicas da Santa Casa de São Paulo
Trabalho realizado na Santa Casa de Misericórdia de São Paulo.
Endereço para Correspondência: Rua Itapeva, 500 10B Bela Vista São Paulo SP 01332-000
Tel (0xx11) 288.5414 - E-mail: quinta@dialdata.com.br
Article submitted on February 19, 2003. Article accepted on February 20, 2003.