Year: 2003 Vol. 69 Ed. 3 - (16º)
Artigo de Revisão
Pages: 394 to 403
Nasopharyngeal Angiofibroma: literature review
Author(s):
Luiz A. C. Ricardo; R
omualdo S. L. Tiago;
Antonio S. Fava
Keywords: -
![]()
INTRODUCTION
Angiofibroma is a benign but very aggressive tumor owing to its tendency to local invasion. Histologically, it is formed by myofibroblasts and vasogenic elements, thus, it is a highly vascularized tumor.
Angiofibromas amount to 0.5% of all head and neck neoplasm cases with an incidence of 1:6,000 to 1:55,000 in the United States1. It seems that there is greater incidence in some parts of the world, such as Mexico, Egypt, southeast of Asia and the Pacific, but no important cause has been identified yet2. There is a clear preference for male subjects, more frequent in adolescence and pre-adolescence.
Macroscopically, the tumor is a globous circumscribed non-encapsulated mass, recovered by rhinopharynx mucosa. The color depends on the vascular component, which can be pinkish-burgundy or pale and whitish. The main blood supply is from the maxillary artery, even though there can be branches of the ipsilateral internal carotid artery or the contralateral external carotid system. Histologically, it is formed by fibrous connective tissue and vessels. The periphery of the tumor presents edema and infiltrates, with necrotic areas and capillary vessels. The deepest vessels are thicker and have more anastomoses, reminding us of the aspect of a cavernous hemangioma. The presence of a single cell layer, without the smooth muscle component, associated with rigidity of the fibrous stroma, conveys the hemorrhagic characteristic to the tumor.
Typically, patients complain of unilateral nasal obstruction and epistaxis without pain symptoms. Additional symptoms, such as diplopia and facial deformity, depend on local extension.
The diagnosis is based on signs, symptoms, morphologic and epidemiological characteristics. Nasal endoscopy, computed tomography and magnetic resonance imaging provide delimitation of the location and extension of the tumor and its relations with vascular and nervous structures, enabling better tumor staging. For this reason and owing to the bleeding characteristic of the tumor, the biopsy is currently not a routine procedure.
Carotid angiography is conducted to study the vascular supply to extensive tumors and to perform embolization to reduce intraoperative bleeding, which is conducted 24 to 48 hours before the surgical procedure.
Surgery and radiotherapy are the main forms of treatment, even though the literature suggests that radical excision with removal of the complete tumor is the preferred therapy. Additional treatment options are embolization, chemotherapy and hormone therapy.
The present article is a review of the terminology and classification of angiofibromas, as well as the progression of the clinical approach, emphasizing therapy. The main objective of the present study is to present in a simple and didactic format the modifications in treatment that have taken place in time according to the literature.
LITERATURE REVIEW
I. Etiopathogenesis
The etiology is still unknown. It is known that there is no correlation with heredity, since no cases of family disease have been reported. The theories include an abnormal response of the nasopharynx bone periosteum connective tissue to a hamartomatous ectopic vascular nucleus, similar to venous lakes that are present in the lower turbinate3, or the aberrant growth of the embryonic occipital lamina before its ossification, which happens at about the age of 25 years.
Up to half of the past century, it was believed that the tumor originated from the embryonic lamina4. It is a cartilaginous structure that originates the basal portion of the occipital bone, sphenoid body, pterygoid medial process, and bones of the lacerus foramen and pterygopalatine space. Up to adolescence and beginning of adult life, the base of the occipital bone is bound to the sphenoid body by a cartilaginous plaque and the perichondrium of this cartilage would be the site of the development of the angiofibroma.
Currently, it is believed that the tumor originates from the posterior-lateral wall of the nasal cavity, where the sphenoid process of the palatine bone finds the horizontal lamina of the vomer and part of the pterygoid process of the sphenoid bone3. This junction forms the superior margin of the sphenopalatine foramen where lies the posterior portion of the middle turbinate (or sphenoid roof)5, 6. This area always seems to be involved, since the lesions irradiated tend to involute in this specific region7. Figure 1 shows schematically the point of origin of the tumor and the expansion routes. Some authors believe that this anomalous tissue growth is caused by elevation of the androgen levels mediated by specific hormone receptors in the tumor tissue5, 8. As of the point of origin of the tumor, there is growth underneath the mucosa extending initially to the posterior portion of the nasal cavity and roof of the rhinopharynx. As the tumor enlarges, it extends through the pterygopalatine foramen, growing laterally to the pterygomaxillary and infratemporal fossae (Figure 2). From this point, it can expand to the soft tissues of the genius region, causing facial bulging, or to the inferior orbital fissure and orbit, causing proptosis, or causing the bone destruction of the base of the pterygoid process, coming into the middle cranial fossa, anterior to lacerus foramen and lateral to the cavernous sinus. The growth keeps on occupying the available space, occupying the nasal cavity and the posterior ethmoid cells, which can directly invade the orbit. Posterior and inferiorly, the tumor bulges the soft palate and can be seen in the oropharynx. Superiorly, the tumor can reach the temporal fossa causing a bulging above the zygoma, or destroy the roof of the sphenoid sinus, occupying it, and it can invade the brain and cause displacement of the hypophysis, sella turcica and optic chiasma.
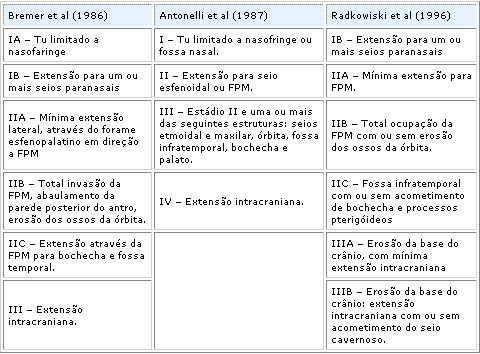
According to Antonelli et al. (1987)9, there are three main routes for cranial invasion: 1) from the infratemporal fossa, through the erosion of the middle fossa floor; 2) through the pterygomaxillary fossa, close to the superior and inferior orbital fissures, and 3) by the superior wall of the sphenoid sinus, reaching the cavernous sinus and/or the pituitarian fossa.
The intracranial invasion is relatively frequent, being described in an incidence of 20 to 36%10. The invasion is caused by bone erosion and displacement of the dura, but it rarely invades it. Butugan et al. (1995)11 in a review of 123 treated cases, found dura tumor invasion in 3 cases, all of them with concomitant invasion of the cavernous sinus, and two of the cases had recurrent disease, in which it is believed that meningeal invasion is more common.
II. Terminology
Angiofibroma is an uncommon tumor of the nose and nasopharynx with firm, fibrous consistency and great vascularization that causes nasal obstruction and epistaxis, being that it can be very severe. There is considerable controversy in the literature about this benign neoplasm, involving terminology and therapeutic approaches.
Reports about the disease seem to data from Ancient Times and many names gave been given to it throughout the years. The most comprehensive review about the topic was made by Harma (1958)12. The author stated that Hippocrates in his writings described the so called hard nasal polyp, probably referring to an angiofibroma. During the 19th century, the tumor was still called fibrous nasal polyp. Bensch (1887) designated it nasopharynx typical polyp to differentiate it from other similar conditions such as antrochoanal polyp and malignant tumors. However, the term polyp was gradually abandoned, giving place to denominations that intended to have more accurate definitions of tumor, such as fibroid basal tumor or simply fibroma. Martin et al. (1948)4 suggested the term juvenile nasopharyngeal angiofibroma. Hubbard (1958) considered that the term juvenile should be abandoned, since the tumor affects also adult subjects. Bremer et al. (1986)13 contested the term nasopharyngeal saying that the tumors clearly expanded beyond the limits of the rhinopharynx, preferring simply to use the term angiofibroma. We should point out at this which are the extrapharyngeal origins of the tumor. Isherwood et al. (1975)14 described a rare case of angiofibroma arising from the skull base, outside the nasopharynx and extending into the pterygomaxillary fossa and the genius region. Rapoport et al. (1977)15 described a case of tongue base angiofibroma in a 22 year-old subject. The most recent publications, though, refer to the tumor as nasopharyngeal angiofibroma.
II. Classification
The importance of staging this tumor has been pointed out as an attempt to perform diagnostic standardization, therapeutic program and result assessment, taking into consideration the high variability in cure and recurrence rates reported in the literature. Conversely, there are multiple staging schemes that have been proposed, hindering the comparison of data.
Sessions et al. (1981)16, based on routes of tumor expansion, proposed a radiological staging considering that the most important element was the number of sites involved rather than the size of the tumor. Staging is as follows: Ia - limited to the nose or nasopharynx; Ib - extension to one or more paranasal sinuses; IIa - minimum invasion to the pterygomaxillary fossa; IIb - complete occupation of the pterygomaxillary fossa, with or without erosion of orbit bones; IIc - invasion of the infratemporal fossa, with or without genius region invasion; III - intracranial extension.
Chandler et al. (1984)7, also based on tumor growth standards and probable site of origin, proposed a system similar to that of the American Joint Committee to nasopharynx cancer: I - tumor confined to nasopharynx; II - extension into the nasal cavity and/or sphenoid sinus; III - extension to one or more of the following structures: maxillary antrum, ethmoid sinus, pterygomaxillary and infratemporal fossae, orbit and/or cheeks, IV - intracranial extension (Table 1).Table 1.
III. Clinical Picture and Diagnosis
Patients in puberty or pre-puberty, male subjects, complaining of uni- or bilateral nasal obstruction, recurrent epistaxis and whose clinical examination reveals the presence of a tumor in the nasal fossa or rhinopharynx, with or without facial bulging, should be investigated for nasopharyngeal angiofibroma. Few isolated cases of angiofibroma in female patients have been reported and its occurrence is so rare that Yan et al. (1998)17 recommended the revision of histology and chromosome study, considering that it could have been a sexual chromosome abnormality combined to female phenotype and karyotype 46XY, such as a male pseudohermaphroditism.
The determination of the age in which the tumor starts its development is very difficult, owing to its predominant location in the nasopharynx, its growth pace and the difficulty to obtain correct information from the patient. Evidently, all symptoms depend largely on the extension of tumors and they may develop for an initial period without clinical manifestations. The mean age of symptoms' onset suggested by various authors ranged from 14 to 16 years12, 13.
Martin et al. (1948)4 determined as 25 mm the diameter of the tumor located in the rhinopharynx that causes nasal obstruction symptoms, but they stated that smaller tumors at the level of the choana can also present the same symptom.
As a result of the advent and the availability of the computed radiological techniques, both the vascular nature of the tumor and its extensions could be shown in three plans and they are extremely important in all stages of the angiofibroma. In fact, owing to the possibility of severe bleeding, many surgeons prefer the imaging methods to biopsy to decide if a nasopharyngeal mass is more probably an angiofibroma than a non-vascular lesion such as an antrochoanal polyp18.
Simple x-rays show the presence of soft tissue in the nasopharynx. Holman and Miller described the antrum signal in profile incidence, which consists of bulging of the posterior wall of the maxillary antrum, showing an invasion of the pterygomaxillary fossa.
The assessment of the tumor extension is made by computed tomography (CT) and magnetic resonance imaging (MRI); the former is used to shows bone abnormalities and the latter, to show the extension to soft tissues. CT scan is the most important exam18. The invasion of the sphenoid sinus is the main predictive factor of recurrence of angiofibroma and the assessment of the presence and extension of the invasion was the main contribution of CT to the diagnosis and treatment of the tumor. The identification of high risk patients for recurrence defined the need to a more radical treatment to eliminate or reduce the size of any tumor remaining in the sphenoid. MRI is used to show the extension of the recurrence, to assess tumor size during radiotherapy and also to monitor a possible natural involution.
The diagnostic angiography frequently fails in defining the tumor and estimating its vascular contents11, and some authors advocate its use only in cases of extensive tumors, especially those involving intracranial invasion, for better therapeutic planning7, 19. Regardless, its routine use was practically abandoned after the use of CT and MRI. The angiogram is more important when we want to conduct selective arterial embolization, a procedure conducted 24 to 48 years before tumor resection in order to reduce intraoperative bleeding.
The biopsy is conducted in the operating room and always via nasal access, owing to the risk of bleeding. However, it is not a routine procedure, since anamnesis data, physical examination and imaging tests practically define the diagnosis, which will be confirmed by the clinical pathology of the operative specimen.
The 24-hour 17-ketosteroid dosage test (steroids, androgens and estrogens) in urine normally does not reveal significant abnormalities.
Many diseases, tumors or not, can cause nasal obstruction, epistaxis and rhinorrhea and can be mistaken by angiofibroma. Among the main differential diagnosis, we can include:
- Inflammatory polyps, normally pedunculated and multiple;
- Nasopharynx malignant tumors - they are rare in adolescence, cause neurological impairment and cervical adenopathy and are normally present in the first visit;
- Adenoid hypertrophy - rarely cause significant nasal bleeding, its aspect is somewhat self-explanatory;
- Angiomatous polyp and nasopharyngeal cyst - special attention should be given to these entities that are easily mistaken by angiofibroma;
- Other entities should be kept in mind: cervical vertebral cordoma, fibrosarcoma and nasopharyngeal fibroma, maxilla malignant neoplasm, nasal fossa esthesioneuroblastoma and retropharyngeal ganglion tuberculosis.
IV. Treatment
Many therapies for angiofibroma have been developed throughout the years, including hormone treatment, chemotherapy, radiotherapy, intra and extracranial surgical techniques, in addition to arterial embolization. The success of such therapies is variable and they are not exempt from side effects, sequelae and possible major complications.
The use of sex hormones for the treatment of angiofibromas refers back to the oldest studies, such as those conducted by Martin et al. (1948)4. Other authors as well as them tried to reduce the rate of tumor growth using estrogen and androgen administration. Farag et al. (1987)20 found in vitro testosterone and dihydrotestosterone specific androgen receptors. Hagen et al. (1994)21 pointed out that the presence of androgen receptors in tumor fibroblasts should be considered unquestionable and clinical application of pure anti-androgen agents (flutamide, ciproterone) in selected cases can represent an alternative to radiotherapy.
Chemotherapy is an alternative treatment for selected cases. Goepfert et al. (1985)6 described two cases treated with doxorubicin and dacarbazin that presented tumor reduction. However, authors recommend caution in its use since there are cases of cardiomyopathy induced by doxorubicin, limiting the use to three specific situations: recurrence after local appropriate therapy, intracranial extension with involvement of vital structures, and blood supply predominantly derived from intracranial vessels.
Surgical approaches for nasopharyngeal angiofibroma have modified throughout the years and many techniques have been used. Hippocrates described one of the first surgeries, which consisted of median nasal incision through which the tumor was removed, but Hippocrates did not mention about success or failure of the surgery.
An issue that has always attracted much interest from investigators, from a therapeutic perspective, is the characteristic of tumor recurrence. This observation led to the development of surgical techniques that allow good exposure of nasopharynx and posterior nasal portions by complete excision of the tumor. Conversely, the multiplicity of the developed surgical techniques, some times quite mutilating ones, attest the inaccessibility of the lesions in this area. The issue is not recent, since there has been for over 150 years a method to approach the nasopharynx through lateral rhinotomy.
Wilson (1951)22 suggested a transpalatine approach, through a transversal incision separating the hard palate from the soft palate, as suggested by Precechtel (1929) for tumor treatment located in the rhinopharynx, describing excellent results and absence of palatal dysfunction of swallowing or phonation. This approach has become the preferred procedure for many surgeons to treat low invasive grade benign or malignant lesions of the nasopharynx and posterior portion of the nasal cavity.
In 1957, the same author 23 published an article describing the various surgical treatment options for rhinopharynx tumors, dividing the procedures in thee main groups:
1. Direct access by the nasal fossa, through different types of external lateral rhinotomy incisions;
2. Transmaxillary access through sublabial incision by the alveolar sulcus or by external rhinotomy approach. At this point, the author reported the incision proposed by Denker, in 1910, as the best sublabial approach, consisting of removing the anterior wall of the maxillary antrum, antrochoanal wall, part of the maxilla frontal process and the nasal bone itself;
3. Transpalatine access, considered by the author as the best approach for the nasopharynx, pointing out the transversal incision of the palate such as the one used by Precechtel for the treatment of choanal atresia.
Cooper (1963)24 published a surgical method for benign and malignant tumor lesions through tissue freezing, the cryogenic surgery. The method is based on the principle that the biological effects caused by freezing are predictable and controllable according to the laws of physics of thermal conduction and that frozen tissues are markedly delimited from adjacent tissues and essentially hemostatic or avascular. English et al. (1972)25 used the Cooper's cryosurgery principle in a series of 12 patients operated through transpalatal surgery, followed by tumor subperiosteal dissection, associated with clamp compression of the external carotid artery. With such technique, the authors emphasized the appropriate homeostasis and easiness to identify the periosteum and tumor resection of the underlying bone tissue.
Sardana (1965)26 suggested the use of a sublabial incision in association with the transpalatal approach to remove the lateral extension of the tumors, pointing out that it is a reasonable option without requiring extensive bone removal, and with the advantage of not causing mutilation, such as in the cases of external rhinotomy and Denker surgery. Conley and Price (1979)27 described degloving with sublabial and transnasal access, with exposure of both maxillary antrums and easiness to approach when compared to other sublabial accesses, such as Caldwell-Luc and Denker incision, in addition to absence of facial incisions.
Maniglia (1986)28 reported his 15-year experience in treating 30 patients with benign or malignant lesions of medium-facial structures, palate or nasopharynx, through medial-facial degloving associated with sublabial incision and rhinoplasty techniques and referred excellent bilateral exposure without requiring external facial deformity.
Euswas (1999)1 published a series of 11 patients classified as stages I to III of Sessions et al., treated by transnasalmaxillary sublabial approach, similar to Denker approach, but without removal of the maxilla frontal process and the nasal bone itself. The procedure, compared to transpalatine approach, lateral and medium-facial rhinotomy, has the advantages of providing wider exposure of the maxillary, ethmoid and sphenoid sinuses, access to the sphenopalatine and internal maxillary arteries, absence of palatal dysfunction risk, absence of facial scar, and easy postoperative assessment by anterior rhinoscopy or endoscopy owing to the large nasosinusal cavity. The author did not detect tumor recurrence in 18 months of follow-up. Some authors, however, consider the lateral rhinotomy approach and its variants as the most direct and versatile route to the tumor body and all its extensions 13, 29.
Briant et al. (1978)30 emphasized the superiority of radiotherapy over surgery in 45 patients. The authors obtained control of the disease in 80% of the cases with a single course of radiation with moderate dose (3,000 to 3,500 rads), being that the remaining 20% reached satisfactory control with one of two additional cycles of radiation; in addition, early side effects were minimum and there was no report of radiation-induced tumor in 20 years of follow-up after therapy. The authors did not inform the staging of treated patients.
Carvalho et. al. (1979)31, in a revision of 29 cases, reported the use of initial surgical treatment using lateral rhinotomy and transpalatine approach in 27.5%, and in the remaining 72.5% the initial treatment was the association of radiotherapy and hormone therapy with testosterone. They emphasized the superiority of this last therapeutic modality, especially in large size tumors. Among other aspects, the authors pointed out that the radio-hormone treatment led to cure in over 50% of the cases, in addition to improving tumor local conditions, in case there is need to proceed surgical intervention.
Fields et al. (1990)8 studied the efficacy of radiotherapy in 13 patients with juvenile nasalangiofibroma during an average period of 136 months. The patients were staged according to the classification by Antonelli et al.9, being 6 patients in stages III and IV, 4 in stage II and 3 in stage I. The authors concluded that owing to the improvement of surgical techniques , improvement in imaging diagnosis and embolization techniques, surgery is the treatment of choice for patients with incipient disease. However, they stated that in patients with extensive lesions (stages III and IV), especially those involving cranial invasion and recurrence, risk/benefit of morbidity and mortality speaks in favor of radiotherapy, recommended in a dose equal or greater than 3,600 cGy.
Gullane et al. (1992)32, studying 21 patients classified according to Chandler et al.7 (1 patient stage II, 17 patients stage III and 3 stage IV), concluded the same, detecting an initial control rate of 64% with surgery and 43% with radiotherapy. New treatment cycles for each modality raise the rates to 93% and 86%, respectively, with an average disease control of 90%. Such values are in compliance with others published in the literature 8, 30.
Other authors, such as Ianetti et al. (1994)12 pointed to surgery as the treatment of choice for such tumors regardless of stage, considering radiotherapy too high risk to malignant transformation.
Reda (1996)33 published a case of juvenile nasoangiofibroma treated with transnasal endoscopy surgery. The author believe that it is a useful approach to treat limited lesions that originate from the sphenopalatine foramen region, with medial extension to the posterior portion of the nasal cavity, posteriorly to the sphenoid sinus and laterally to the pterygopalatine fossa (Figure 1), with no need to incise the palate, sublabial or external region. However, they recognized the limited experience once there was just one treated case.
DISCUSSION
Angiofibroma is a rare tumor with benign histology characteristics but it has a behavior comparable to that of malignant tumors, owing to its tendency to grow invasively and expansively, destroying the bone plans and invading the paranasal sinuses, temporal fossae and skull base, in addition to frequent hemorrhagic episodes, which are massive and potentially fatal.
Despite the considerable controversy in the literature, most authors prefer to refer to the tumor as a nasopharyngeal angiofibroma and the main staging systems used are those by Sessions et al. (1981)16 and Chandler et al. (1984)7, based respectively on the expansion routes and growth pattern and probable site of origin.
The clinical picture does not offer great difficulties provided that the attending physician bears in mind the possibility of treating an angiofibroma. Computed tomography increases diagnostic capabilities and safely defines the extension of the tumor, being the most important complementary test. Its main contribution is the assessment of the sphenoid invasion, which is the main predictive factor of tumor recurrence, predicting the need for a more radical approach.
Some authors in the past suggested that the tumor could present spontaneous resolution after puberty 4, 12. but the phenomenon has been rarely documented in the literature and the wait and see approach is currently considered excessively optimistic and some form of therapy should be practiced, given the infiltrate and destructive characteristic.
The almost exclusive occurrence in the male gender and its origin has always been obscure in the pathophysiology of the tumor. Some authors 4, 12 suggested exclusive affection in males and Martin has even stated that it was a sex-linked tumor. Hormone dosages and the study of secondary sexual characteristics did not support this understanding of the issue, because in general patients present sexual development within the expected age range. However, there is evidence that tumor behavior varies with the variation of hormone serum levels. Schiff34, in his thesis about the pathogenesis of the angiofibroma, described in details the presence of tumor typical immature fibroblasts and endothelial cells and emphasized that the clinical picture is drastically reverted by estrogen action, which causes histology maturation of the connective tissue and endothelial cells. However, Schiff did not identify the potential origin of the tumor.
Johnson et al. (1966)29 observed accelerated growth after the application of testosterone and reduction of the tumor as a result of estrogen use. Hagen et al. (1999)21 and Farag et al. (1987)20 demonstrated the presence of dihydrotestosterone receptors in tumor fibroblasts, explaining its growth through the mediated response of specific receptors, being hyperandrogenism in male puberty a stimulus for the development of the angiofibroma. If on the one hand the presence of androgen receptors is considered a confirmed fact 12, different studies in the literature detected the absence of estrogen receptors 9, 35-36, suggesting an indirect action of the hormone. Schiff34 suggested a double mechanism of estrogen action: reduction of permeability of connective tissue, reducing the number of vessels, and reduction of androgen production by negative feedback. The findings of the authors pointed to the possibility of using pure antiandrogen agents (flutamide, ciproterone) as an alternative to radiotherapy and chemotherapy treatments. However, such substances are considered potential alternatives in extensive tumors 21 and the success of the hormone treatment is not a constant finding in the literature. The issue is probably even wider, as posed by some authors 2, 31 which did manage to have tumor reduction but not with antiandrogen or estrogen agents, but rather with the use of male hormone (testosterone propionate). Regardless, isolated hormone therapy seems to have no curative effect, being considered always as an auxiliary therapy to surgery or radiotherapy 2.
In the past years, more importance was given to the possible action of the growth factors acting in the tumor extracellular matrix, leading to its progression. Schiff et al. (1992)37 found enough amounts of bFGF (basic fibroblast growth factor) in tumor extracts through the techniques of immunoassay. It is a mitogenic agent for endothelial cells, smooth muscle cells and fibroblasts. In Brazil, Nagai et al. (1996)38 studied the expression of mRNA in various growth factors possibly involved in the proliferation of mesenchymal cells, suggesting that IGF-II (insulin-like growth factor-II) could be a potential regulator of nasopharynx angiofibroma growth.
Based on the principle that tumor growth is determined by a specific response mediated by a receptor, the proposal by Yang et al.17 of conducting chromosome study in female patients to try to find invisible chromosome abnormalities did not seem to be feasible, since we are facing chromosome abnormalities in the female phenotype and karyotype 46XY, which would not justify the occurrence of the tumor, since the subjects presented resistance to the peripheral action of the androgen or even of its synthesis.
Chemotherapy remains an alternative for difficult to treat cases, but we recommend caution in its indication, especially concerning toxicity, since the results of this type of treatment are still preliminary.
Surgery and radiotherapy seem to be the more effective treatment 13. Most authors, however, point to radical surgery as the most effective form of treatment. The literature shows that both in patients with initial surgical treatment or radiotherapy, the rates for final control of the disease are similar (80%), even though the patients had needed subsequent treatment for recurrence with the association of such methods 8, 30.
Surgical resection varies from nasal endoscopy techniques to open techniques combined with intra- and extracranial approach, including lateral, sublabial and transpalatal rhinotomy, either associated or not. The surgical technique to be employed depends on the extension of the lesion and we should always observe the principles of good exposure of the surgical field and level of radical approach and it has better results in more delimited lesions that have not invaded the cranial region.
The surgical procedure is always more likely to produce severe hemorrhage that can take to patients' death. Ligation of the external carotid artery has been tried to prevent bleeding, but it was later considered useless owing to collateral circulation and nasopharynx anastomosis and predominance of venous circulation of the tumor. Intraoperative tumor freezing (cryosurgery) as practiced by English et al. (1972)25, reduces bleeding and facilitates handling of the tumor. The main criticism concerning the procures, however, is its limitations with large lesions, since freezing does not reach all extension of tumors that infiltrate the muscles and bones. In smaller lesions, this approach would be effective, but in such cases they can be removed without cryotherapy, with an acceptable level of bleeding. The most effective and currently used technique is selective arterial embolization, leading to reduction in surgical blood loss and facilitating the excision. The major complication for this procedure is emboli leak to the intracranial circulation, but it is a rare occurrence and does not contraindicate the procedure. Other minor complications, associated with embolization are: systemic reaction to contrast, infection on the punch site, hematoma and femur thrombosis. Even though arterial embolization reduces intraoperative bleeding, it can increase the risk of recurrence, especially when there is deep invasion of the sphenoid, because it makes total removal more difficult. However, patients that show this invasion the preoperative CT scan are under high risk of recurrence and embolization is contraindicated 39.
After Wilson (1951)23 suggested a transpalatine surgical approach with palate transversal incision, similarly to Precechtel, in 1929, for the treatment of choanal atresia, this technique became more popular with the surgeons at the time, and the main advantages were no external incision and the fact that it promoted an access route directed to the sphenoid and posterior ethmoid cells (Figure 3). Some suggested association of the transpalatal approach with rhinotomy 25 and sublabial incisions 26 for the treatment of tumor extensions, especially in the genius region. However, for some authors the transpalatine access was considered restricted. Cocke, in 1964 (apud Conley and Price 197327), believed that it could not be performed with tumors larger than 5 cm, and even so it could lead to significant palatal dysfunction.
Sublabial incision also play a key role. It is difficult to define in the literature who first idealized the access. It is known that Rouge and Halsted, in 1870, described the operations in which sublabial incisions were used, extending from one canine fossa to the other, with upper lip eversion, elevation of the nose ala together with the underlying periosteum, and division or partial removal of the nasal septum. In 1910, Denker proposed a sublabial incision to access the nasopharynx, with unilateral exposure of the maxillary antrum considered by Wilson22 as the only useful sublabial approach. During the second half of the century, many authors emphasized the importance of having the sublabial approach. Casson and Converse (apud Lloyd et al. 199918) also described degloving techniques to expose the medium-facial skeleton upon treating 50 patients with different pathologies. Conley and Price (1979)27 described the degloving emphasizing the advantage of exposing both maxillary antrum and the easy access to the pterygoid palatine space, when compared to the transpalatine access, Caldwell-Luc and sublabial Denker. Maniglia (1986)28 proposed the degloving as a replacement to lateral rhinotomy and Lloyd (1999)18 considered the midfacial degloving the preferred treatment for nasopharynx angiofibroma.
Lateral rhinotomy and its variations, as pointed out by Bremer et al. (1986)13, provided excellent exposure and versatility concerning the approach of the tumor and its extensions, but it has the inconvenience of producing a large scar on the facial medium third.
Recently, the endoscopic approach has emerged as a surgical option to treat these tumors. The advances in this area enabled the use of trasnasal and transoral endoscopic procedures for angiofibromas. Transnasal endoscopic approach is best indicated for small tumors, limited to the nasopharynx, nasal cavity, ethmoid and sphenoid sinuses, and it can be tried in tumors that extend up to the pterygopalatine fossa 5. The main advantages are reduced destruction of soft and bone tissues and absence of palate, sublabial or external incisions. The endoscopic nasal techniques can also be used together with other approaches to assess the extent of tumor and sufficiency level of the resection. Conversely, the surgical field is restricted and bleeding becomes a major problem, being essential to perform embolization of the main vessels that supply the tumor. Further experience with the use of endoscopic techniques is required but they have been consolidated as a surgical treatment option for more limited lesions.
The best surgical option is not a consensus yet, and obviously it depends on the experience of each surgeon. We should agree with Fagan et al. (1997)5 proposition, a published experience with 16 surgically treated patients, which considered that the surgical approach depended on the extension of the disease. Therefore, they used transnasal endoscopic approach in cases in which the disease did not extend beyond the pterygopalatine fossa. The medial maxillectomy, through medial facial access (degloving) or external rhinotomy incisions, was recommended for lesions that affected the medial portions of the infratemporal fossa and cavernous sinus.
The treatment of cases with significant involvement of the infratemporal fossa and cavernous sinus, or large mass on the skull base, gave origin to the greatest divergence among authors 19. The management varies from authors that believe that if the tumor invades the intracranial region they are irresectable, to authors that advocate a combined intra and extracranial approach, including also those authors that propose resection only of the extracranial tumors and complementation of the treatment with various methods, such as radiotherapy, chemotherapy, hormone therapy, embolization, etc 19.
What seems to be well established in the literature concerning surgical treatment of tumors with intracranial extension is that it is better not to risk a complete resection of a localized disease in the cavernous sinus and/or pituitary fossa, preferring postoperative adjuvant therapy 11, 19.
Radiotherapy, in turn, has the best results in damages with intracranial extension or recurrent lesions. The first caution is the radiation of benign lesions, with the risk of inducing malignant transformation. The estimate in the literature is that the risk of malignancy is four times greater in patients submitted to radiation than in the general population 30 and the risk/benefit should be considered individually, recommending a moderate dose (3,500 rads). Other possible effects of radiotherapy are dryness of the jugal and nasal mucosa, leading to tooth decays and atrophic rhinitis with formation of crusts, interference in scarring process, in addition to the effects of the facial growth centers, relevant to the age range of these patients. However, in the studied literature, such effects were dealt with and none of them were significant.
Recurrence is a constant concern in such patients and the literature reports incidences that range from 6% to 24% 9, 32. The absence of a tumor capsule, hindering surgical cleavage and the tendency to disseminate into the submucosa are the main causes of recurrence of the surgically treated cases 5. Failure of radiotherapy treatment is normally attributed to the so-called "geographical losses" in the area of radiation and insufficient doses. The presence of a tumor in the sphenoid is the main predictive factor of recurrence in both forms of treatment, either surgical or radiotherapy.
CLOSING REMARKS
In our opinion and based on the studied literature, there are still no absolute facts about the treatment of angiofibromas. However, we noticed a tendency to choosing selective arterial embolization, followed by radical surgery as the best form of treatment. Radiotherapy has a role to play in cases in which the tumor mass has intracranial invasion, especially in the dura, cavernous sinus and pituitary fossa, in which complete surgical resection is difficulty and risky, or in cases of recurrence. The other therapeutic approaches such as hormone therapy and chemotherapy are controversial options and they are still potential alternatives to the treatment of angiofibromas.
REFERENCES
1. Euswas C. A result of sublabial transnasomaxillary approach of nasopharyngeal angiofibroma: retrospective study. J Med Assoc Thai 1999;82:234-41.
2. Butugan O. Angiofibroma Juvenil - Aspectos Atuais Terapêuticos. In Brandão LG, Ferraz AR. Cirurgia de Cabeça e Pescoço. Brasil: Editora Roca; 1989. Vol. 2. p. 415-20.
3. Neel HB, Whicker JH, Devine KD, Weiland L H. Juvenile angiofibroma. Am J Surg 1973;126: 547-56.
4. Martin H, Ehrlich HE, Abels JC. Juvenile nasopharyngeal angiofibroma. Ann Surg 1948;137:513-35.
5. Fagan JJ, Snyderman CH, Carrau RL, Janecka IP. Nasopharyngeal angiofibromas: selecting a surgical approach. Head Neck 1997;19:391-9.
6. Goepfert H, Cangir A, Lee Y. Chemotherapy for aggressive juvenile nasopharyngeal angiofibroma. Arch Otolaryngol 1985;111:285-9.
7. Chandler JR, Moskowitz L, Goulding R, Quenceb RM. Nasopharyngeal angiofibromas: staging and management. Ann Otol Rhinol Laryngol 1984;93:322-9.
8. Fields JN, Halverson KJ, Devineni VR. Juvenile Nasopharyngeal Angiofibroma: Efficacy of Radiation Therapy. Radiology 1990;176: 263-5.
9. Antonelli AR, Cappiello J, Donajo CA Lorenzo D Nicolai P Orlandini B. Diagnosis, staging and treatment of juvenile nasopharyngeal angiofibroma. Laryngoscope 1987;97:1319-25.
10. Close L. Surgical Management of Nasopharyngeal Angiofibroma Involving the Cavernous Sinus. Arch Otolaryngol Head Neck Surg 1989;115: 1091-95.
11. Butugan O, Sennes L U, Almeida ER, Miniti A. Angiofibroma Juvenil Intracraniano com Invasão Meníngea e de Seio Cavernoso. Revista Brasileira de Otorrinolaringologia 1995;61: 400-12;.
12. Harma RA. Nasopharyngeal angiofibroma Acta Otolaryng suppl 1958;146: 1-76.
13. Bremer JW, Neel HB, Desanto LW Jones GC. Angiofibroma treatment trends in 150 patients during 40 Years. Laryngoscope 1986;96:1321-8.
14. Isherwood I, Dogra TS, Farrington WT. Extranasopharyngeal juvenile angiofibroma. J Laryngol Otol 1975;89:535-44.
15. Rapoport A, Andrade Sobrinho J, Carvalho MB, Giannotti Filho O, Scandiuzzi D. Angiofibroma Juvenil de Língua: relato de um caso. Revt Bras Cirurg 67 1977;339-343.
16. Sessions B B, Bryan B, Naclerio R, Alford B. Radiographic staging of juvenile angiofibroma. Head And Neck Surg 1981;3;279-283.
17. Yang P-W, Sheen T-S, Ko J-Y, Liu HM, Hsu MM. Nasopharyngeal angiofibroma: a reappraisal of clinical features and treatment at National Taiwan University Hospital. J Formos Med Assoc 1998;97:845-9.
18. Lloyd G, Howard D, Phelps P, Cheesman A. Juvenile angiofibroma: the lessons of 20 years of modern imaging. J Laryngol Otol 1999;113:127-33.
19. Granato L. Angiofibroma Juvenil - Vias de Abordagem, Técnicas e Complicações. In: Brandão LG, Ferraz AR. Cirurgia de Cabeça e Pescoço, Brasil: Editora Roca; 1989. Vol 2. p. 397-414.
20. Farag MM, Ghanimah SE, Ragie A. Hormonal Receptor in Juvenile Nasopharyngeal Angiofibroma. Laryngoscope 1987;97;208-211.
21. Hagen R, Romalo G, Schwab B, Hoppe F, Schweikert HU. Juvenile nasopharyngeal fibroma: androgen receptors and their significance for tumor growth. Laryngoscope 1999;104:1125-9.
22. Wilson CP. The approach to the nasopharynx. Proc R Soc Med 1950;44:353-8.
23. Wilson CP. Observations on the surgery of the nasopharynx. Ann Otol Rhinol Laryngol 1957;66:5-27.
24. Cooper IS. Cryogenic surgery: a new method of destruction or extirpation of benign or malignant tissues. N Engl J Med 1963;268:743-8.
25. English GM, Hemenway WG, Cundy RL. Surgical treatment of invasive angiofibroma. Arch. Otolaryngol 1972;96:312-8.
26. Sardana DS. Nasopharyngeal fibroma. Arch Otol 1965;81:584-8.
27. Conley J, Price JC. Sublabial approach to the nasal and nasopharyngeal cavities. Am J Surg 1979;138:615-8.
28. Maniglia AJ. Indications and techniques of midfacial degloving. Arch Otolaryngol Head Neck Surg 1986;112: 750-2.
29. Johnson S, Kloster J, Schiff M. The action of hormones on juvenile angiofibroma. A case report. Acta Otolaryngol 1966;61;153-160.
30. Briant TDR, Fitzpatrick PJ, Berman J. Nasopharyngeal angiofibroma: a twenty year study. Laryngoscope 1979;88:1247-51.
31. Carvalho MB, Andrade Sobrinho J, Rapoport A, Fava AS, Magrin J, Scandiuzzi D. Angiofibroma juvenil da nasofaringe. Rev Paul Med 1979;93;52-62.
32. Gullane PJ, Davidson J, O'dwyer T, Forte F. Juvenile angiofibroma: a review of the literature and a case series report. Laryngoscope 1992;102:928-33.
33. Reda HK. Transnasal endoscopic surgery in juvenile nasopharyngeal angiofibroma. J Laryngol Otol 1996;110:962-968.
34. Schiff M, Gonzalez AM, Ong M, Baird A. Juvenile Nasopharyngeal Angiofibroma Contain an Angiogenic Growth Factor: Basic FGF. Laryngoscope 1992;102: 940-45.
35. Brentani MM, Butugan O, Oshima CTF, Torloni H, Paiva LJ. Multiple Steroid Receptors in Nasopharyngeal Angiofibromas. Laryngoscope 1989;99: 398-401.
36. John ME, Macleod RM, Cantrell RW. Estrogen Receptors in Nasopharyngeal Angiofibromas. Laryngoscope 1980;90: 628-34.
37. Schiff M. Juvenile nasopharyngeal angiofibroma. A theory of pathogenesis. Laryngoscope 1959;69;981-1013.
38. Nagai MA, Butugan O, Logullo A, Brentani MM. Expression of Growth Factors, Proto-oncogenes and p53 in Nasopharyngeal Angiofibromas. Laryngoscope 1996;106: 190-95.
39. Lloyd G, Howard D, Lund VJ Savy L. Imaging for juvenile angiofibroma. J Laryngol Otol 2000;114:727-30.
40. Doyle PJ. Approach to tumors of the nose, nasopharynx and paranasal sinuses Laryngoscope 1968;78: 1756-62.
41. Finerman WB. Juvenile Nasopharyngeal Angiofibroma in the Female Arch Otolaryngol 1951;54;620-623.
42. Ianetti G, Belli E, De Ponte F, Cicconetti A, Delfini R. The surgical approaches to nasopharyngeal angiofibroma. J Cranio Maxillo-Facial Surg 1994;22:311-6.
43. Kamel RH. Transnasal endoscopic surgery in juvenile nasopharyngeal angiofibroma. J Laryngol Otol 1996;110:962-8.
Master studies in Otorhinolaryngology under course, IAMSPE (Instituto de Assistência Médica ao Servidor Público Estadual de São Paulo).
Master in Otorhinolaryngology and Head and Neck Surgery, Ph.D. studies at UNIFESP-SP. Assistant Physician of Head and Neck Surgery, Department of Otorhinolaryngology, HSPE-FMO, São Paulo. Assistant physician of the Service of Otorhinolaryngology, Hospital do Servidor Público Municipal (HSPM) São Paulo.
Ph.D. in surgery, Medical School, University of São Paulo. In Charge of the Service of Head and Neck Surgery, Department of Otorhinolaryngology, Hospital do Servidor Público Estadual - FMO, São Paulo.
Affiliation: Hospital do Servidor Público Estadual (HSPE)
Address correspondence to: Luiz A. C. Ricardo. Rua Coronel Camisão, 362 - Butantã, São Paulo.
CEP: 05590-201. Tel. (55 11) 3726-1442. E-mail: arturcostarica@ig.com.br
Article submitted on May 02, 2002. Article accepted on July 18, 2002.
All rights reserved - 1933 /
2025
© - Associação Brasileira de Otorrinolaringologia e Cirurgia Cérvico Facial