Year: 2006 Vol. 72 Ed. 5 - (12º)
Artigo Original
Pages: 654 to 658
GSTT1 and GSTM1 polymorphism in cigarette smokers with head and neck squamous cell carcinoma
Author(s): Joice Matos Biselli1, Renata Cristina de Angelo Calsaverini Leal2, Mariângela Torreglosa Ruiz3, Eny Maria Goloni-Bertollo4, José Victor Maníglia5, Andréa Regina Baptista Rossit6, Érika Cristina Pavarino-Bertelli7
Keywords: glutathione transferase, head and neck neoplasms, polymorphism, tobacco, alcohol.
Abstract:
Gene variability related to carcinogen activation and detoxification may interfere with susceptibility to head and neck cancer. Aim: To investigate the relation between GSTT1 and GSTM1 null polymorphisms and the risk of head and neck squamous cell carcinoma in cigarette smokers. Material and Method: A case-control study conducted at the Sao Jose do Rio Preto Medical School, Brazil. GSTM1 and GSTT1 null genotype frequencies were evaluated by multiplex PCR in 45 cigarette smokers with head and neck squamous cell carcinomas and 45 cigarette smokers without this disease. Results: The oral cavity was the most prevalent tumor site for squamous cell carcinoma. The GSTT1 null genotype was found in 33.3% of the Experimental Group and 23.3% of the Control Group (p= 0.311). Experimental and Control Groups had GSTM1 null genotype frequencies of 35% and 48.3% (p=0.582). No association between alcohol consumption and GSTT1 and GSTMI null genotypes was found in these groups (p-values>0.05). There were more men, and alcohol consumption was prevalent in both groups. Conclusion: In this study we were unable to show a correlation between GSTM1 and GSTT1 genotypes and the development of head and neck squamous cell carcinomas in cigarette smokers.
![]()
INTRODUCTION
Head and neck neoplasms are responsible for many deaths worldwide, being the sixth cause of death by câncer.1 The most frequent histological type is the squamous cell carcinoma, which is found in over 90% of cases,2-4 and is usually associated with the use of alcohol and tobacco.5-9 It is known that cigarette smoke is a complex mixture of over 4,000 substances, of which at least 40 are carcinogenic, initiating or promoting tumors in animals.10 Levels of electrophilic products launched into the bloodstream depend on the action of enzymes involved in biometabolism, which includes activation (Phase I) and detoxification (Phase II) of chemical compounds.10,11 Polymorphisms in genes that codify these enzymes may alter their expression or function, altering carcinogenic compounds biometabolism12.
Various polymorphic genes that code enzymes involved in carcinogen biotransformation have been associated with cancer development.13-22 Two genes in particular - GSTT1 and GSTM1 - that code phase II enzymes belonging to the glutathione S-transferases (GSTs) family seem relevant for susceptibility to head and neck squamous cell carcinoma; they detoxify carcinogenic tobacco smoke reactive metabolites.11-12,13,15,18,20,23
The GSTM1 gene is polymorphic in humans, including a null-activity allele (GSTM1-) due to a major genic deletion, and two functional alleles (GSTM1A and GSTM1B). The GSTT1 gene is also polymorphic in humans, and may have a deletion null genotype.24,25 Individuals that have one of these null genotypes in homozygosis may be grouped into the negative conjugating phenotype, where there is complete loss of enzyme activity,26,27 while those that have at least one functional allele are grouped in the positive conjugating phenotype.28
Thus, individual gene variability in the metabolic activation and detoxification process appears to be crucial to head and neck cancer susceptibility.
This study aimed to identify GSTT1 and GSTM1 gene null genotypes in smokers with head and neck squamous cell carcinoma and to compare these frequencies with those seen in smokers without a history of cancer, to identify possible susceptibility biomarkers for head and neck cancer.
MATERIAL AND METHODS
Individuals diagnosed with squamous cell head and neck carcinomas confirmed by histopathology came from the Department of Otorhinolaryngology and Head and Neck Surgery of the Navy Hospital / Medical School of São José do Rio Preto and the Arnaldo Vieira de Carvalho Institute, SP. The control group included individuals with no history of neoplastic disease, paired by gender, age, ethnic group and use of alcoholic beverages. All subjects (patients and controls) were smokers. Individuals were included in the study after signing a free and informed consent form, and all information was obtained using a confidential standardized data-collection questionnaire (gender, ethnic group, smoking and use of alcohol). Information on smoking and alcohol-drinking was limited to a definition of user or non-user. The study was approved by the Research Ethics Committee of the Sao Jose do Rio Preto Medical School (CEP-FAMERP - 5639/2002) and the National Research Ethics Council (CONEP - legal process nº 842/2003).
Genomic DNA was extracted from peripheral blood according to the Abdel-Rahman et al technique.29 A sample of peripheral blood was collected in a test tube containing anticoagulant (EDTA) and lymphocytes were isolated using the Ficoll-Paque Plus. Genomic DNA was obtained by adding SDS (Sodium Dodecyl Sulphate), proteinase K and RNAse A to the isolated lymphocytes. Following NaCl purification, DNA was precipitated with ethanol and stored at -20ºC in a Tris-EDTA buffer solution for later analysis.
The analysis of GSTT1 and GSTM1 genes was simultaneously done by the chain reaction (PCR) according to Abdel-Rahman et al.30 Amplification of the DNA sequence of interest was obtained in 35 cycles that included DNA denaturing steps at 94ºC for 2 minutes, annealing of reaction-initiator sequences (primers) at 59ºC for 1 minute, and DNA chain extension by nucleotide addition at 72ºC for 1 minute. A CYP1A1 gene exon 7 sequence was co-amplified to serve as an internal amplification control. PCR products were analyzed in agarose gel at 1.5%, stained with ethydium bromide, and the null genotype (both alleles with a deletion) for the GSTT1 and GSTM1 genes was identified by the absence of amplification fragments of 480 base pairs (bp) and 219 bp respectively. Presence of the 312 bp fragment corresponded to the amplified CYP1A1 gene sequence, which was evidence of a successful amplification reaction.
Demographics were presented as mean ± standard deviation (SD) or proportions. For the statistical analysis of the genotype frequencies obtained we used the exact Fisher test, with a significance level below 5%.
RESULTS
Demography data: 120 individuals were recruited, of which 60 had head and neck squamous cell carcinoma (average age 54.6 ± 8 years) and 60 had no history of neoplastic disease (average age 54 ± 9 years). Men predominated (90% men vs. 10% women) and there were more alcohol drinkers (70% alcoholics vs. 30% non-alcoholics).
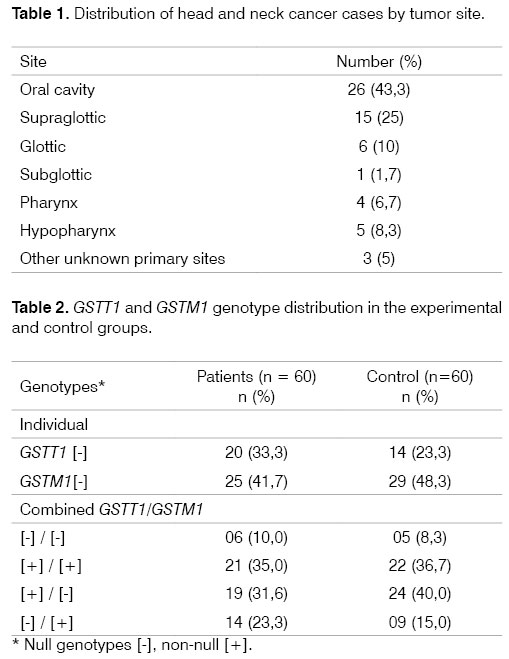
Primary sites: all head and neck squamous cell carcinoma cases were diagnosed and confirmed by pathology exams. The distribution of the primary tumor site is shown on Table 1. Mouth cancers were the most frequent type.
Frequency of polymorphisms: The GSTT1 null genotype [-] was found in 33.3% (20 of 60) of patients and in 23.3% (14 of 60) of controls (P = 0.311). The GSTM1 null genotype [-] was found in 21 (35%) of patients and 29 (48.3 %) of controls (p = 0.582). The combined GSTT1 and GSTM1 gene null genotype was seen in 10% (6 of 60) of patients and in 8.3% (5 of 60) of controls (p = 1.0). The most frequent genotypic combination, considering the presence of an unfavorable genotype (null GST), was GSTT1 [+] / GSTM1 [-] in 31.6% (19 of 60) of patients and 40% (24 of 60) of controls (p = 0.353) (Table 2).
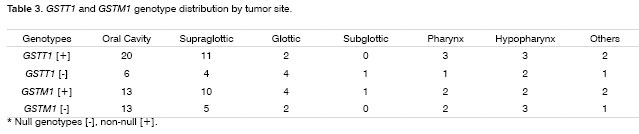
Genotypes were grouped by tumor site (Table 3) and there was no correlation with GSTT1 and GSTM1 null genotypes.
Statistical analysis did not reveal any relation between alcohol use and GSTT1 and GSTM1 null genotypes [-] when we compared alcohol-drinking patients and controls (GSTT1, p = 0.34; GSTM1, p = 0.28; GSTM1[-] / GSTT1[-], p = 1.0).
DISCUSSION
Epidemiological data has suggested that alcohol use and smoking are the main risk factors for malignant transformation in head and neck cancers.31,32 It is difficult to assess the individual effect of these agents on the genesis of head and neck cancer. Smokers tend to consume alcohol and alcohol drinkers tend to smoke. Furthermore, in past decades the evidence has show that both factors operate synergistically, and as such may be the main cause of tumors.32
Studies have shown an association between alcohol drinking and the development of head and neck tumors when taking into account exposure time and the amount of alcohol consumed.12,34 In our study, 70% of patients with cancer drink alcoholic beverages, which strengthens the association between alcohol use and the development of head and neck cancer. This is especially true in a sample that includes smokers. Unfortunately we were unable to obtain information about the amount of alcohol that was consumed or the exposure time.
The higher number of men in this study corroborates findings by Drummond et al,22 although our case number is not sufficient to explain this gender difference. An epidemiological study done at the A. C. Camargo Hospital in the state of Sao Paulo, Brazil, showed that the predominance of men in patients with mouth cancer appears to reflect differences in alcohol use and smoking between men and women in this country.35
The most frequent tumor site in our patients was the mouth, which is also the most frequent site for head and neck tumors reported in literature.36 This anatomical distribution may be explained by the fact that our sample was made up of smokers, most of which also consumed alcoholic beverages, reflecting the fact that the upper aerodigestive tract is more directly exposed to tobacco and alcohol.
GSTT1 and GSTM1 polymorphism studies done in Brazilian sub-populations reveal similar frequencies for both genotypes. Rossit et al,37 in a study among populations from the states of Para and Sao Paulo, showed frequencies of 18 and 47.3% for GSTT1 and GSTM1 gene null genotypes, respectively. Rossini et al,38 revealed frequencies of 25.4% for GSTT1 [-] and 42.1% for GSTM1 [-] in the state of Rio de Janeiro. Our study showed similar frequencies for these genotypes (24.4 and 17.8% for GSTT1 [-] in patients and controls, and 44.4 and 48.9% for GSTM1 [-] in patients and controls). Higher GSTT1 [-] and GSTM1 [-] polymorphism frequencies were observed by Drummond et al.21,22 in Brazilian smokers with oral cavity squamous cell carcinoma (81.8% for GSTT1 [-] and 70.5% for GSTM1 [-]).
GSTT1 and GSTM1 polymorphism studies done in head and neck carcinomas are contradictory. Various authors have demonstrated an association with the GSTM1 null genotype [-],14,15,20,21,38,40,41 which other authors have not seen.5,16,42,43 A relation with the GSTT1 null genotype [-] was also shown in some studies,15,20,22,44 which was not seen in other studies.5,16,37,39,41,43,46 The combination of these higher risk and null genotypes has also been observed in this type of carcinoma.14,41 Moreover, both exposure time and intensity to carcinogenic (alcohol and tobacco) may influence on the interaction of these genotypes for the development of this kind of neoplasia12,34, and these data were not considered in our study. A study by Konig-Greger et al47 showed that GSTM1-enzyme activity was significantly reduced in patients with head and neck carcinoma compared to controls, although it did not depend on the unfavorable GSTM1 genotype, which may suggest that other enzymes participate as regulators.
Interestingly, Evans et al13 found that the positive GSTT1 genotype [+] is associated with an increased risk of head and neck squamous cell carcinoma in smokers (OR = 1.6; CI 95% = 1.1-2.5) and that the GSTT1 null genotype [-] may protect individuals against the development of this cancer. Although usually GSTs are considered detoxification enzymes, for certain specific chemical substrates such as dichloromethane (DCM), conjugation of glutathione with the GSTT1 enzyme may activate an electrophilic component, resulting in mutagenic potential.48,49 Although DCM is not associated with tobacco, tobacco byproducts may gain carcinogenicity by GSTT1-enzyme mediated activation. Two other papers have also shown an increased risk of coronary disease and peripheral vascular disease in smokers with a GSTT1 genotype [+].50,51
CONCLUSION
This study did not allow us to establish any correlation between GSTT1 and GSTM1 null genotypes and the development of head and neck squamous cell carcinoma in smokers.
ACKNOWLEDGEMENTS
We would like to acknowledge Professor Doctor Eloiza Helena Tajara for her contributions to this paper.
REFERENCES
1. Walker DM, Boey G, McDonald, LA. The pathology of oral cancer. Pathology 2003;35(5):376-83.
2. Casiglia J, Woo SB. A comprehensive review of oral cancer. Gen Dent 2001;49(1):72-82.
3. Reichart PA. Identification of risk groups for oral precancer and cancer preventive measures. Clin Oral Investig 2001;5(4):207-13.
4. Ahmed KA, Robbins KT, Wong F, Salazar JE. Efficacy of concomitant chemoradiation and surgical salvage for N3 nodal disease associated with upper aerodigestive tract carcinoma. Laryngoscope 2000;110(11):1789-93.
5. Chang HW, Ling GS, Wei WI, Yuen AP. Smoking and drinking can induce p15 methylation in the upper aerodigestive tract of healthy individuals and patients with head and neck squamous cell carcinoma. Cancer 2004;101(1):125-32.
6. Hasnis E, Reznick AZ, Pollack S, Klein Y, Negler RM. Synergistic effect of cigarette smoke and saliva on lymphocytes - the mediatory role of volatile aldehydes and redox active iron and possible implications for oral cancer. Int J Biochen Cell Biol 2004;36(5):326-839.
7. Kjaerhim K, Gaard M, Andersen A. The role of alcohol, tobacco, and dietary factors in upper aerogastric tract cancers: a prospective study of 10.900 Norwegian men. Cancer Causes and Control 1998;9(1):99-108.
8. Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, Bernstein L, Schoenberg JB, Stemhagen A, Fraumeni JF Jr. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res 1988;48(11):3282-7.
9. Llewellyn CD, Linklater K, Bell J, Johnson NW, Warnakulasuriya S. An analysis of risk factors for oral cancer in young people: a case-control study. Oral Oncol 2004;40:304-13.
10. Maser E. Stress, hormonal changes, alcohol, food constituents and drugs: factors that advance the incidence of tobacco smoke-related cancer? TIPS 1997;18(8):270-5.
11. Anwar WA, Abdel-Rahman SZ, El-Zein RA. Genetic polymorphism of GSTM1, CYP2E1and CYP2D6 in Egyptian bladder patients. Carcinogenesis 1996;17(9):1929-32.
12. Olshan, AF, Weissler MC, Watson MA, Bell DA. GSTM1, GSTT1, GSTP1, and NAT1 polymorphisms, tobacco use, and the risk of head and neck cancer. Cancer Epidemiol Biomakers Prev 2000;9(2):185-91.
13. Evans AJ, Henner WD, Eilers KM, Montalto MA, Wersinger EM, Andersen PE et al. Polymorphisms of GSTT1 and related genes in head and neck cancer risk. Head Neck 2004;26(1):63-70.
14. Gronau S, Koenig-Greger D, Jerg M, Riechelmann H. Gene polymorphism in detoxification enzymes as susceptibility factor for head and neck cancer? Otolaryngol Head Neck Surg 2003;128(5):674-80.
15. Amador AG, Righi PD, Radpour S, Everett ET, Weisberger E, Langer M et al. Polymorphisms of xenobiotic metabolizing genes in oropharyngeal carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;93(4):440-5.
16. To-Figueras J, Gené M, Gómez-Catalán J, Piqué E, Borrego N, Caballero M et al. Microsomal epoxide hydrolase and glutathione S-transferase polymorphism in relation to laryngeal carcinoma risk. Cancer Lett 2002;187(1-2):95-101.
17. Jourenkova-Mironova N, Voho A, Bouchardy C, Wikman H, Dayer P, Benhamou S et al. Glutathione S-transferase GSTM3 and GSTP1 genotypes and larynx cancer risk. Cancer Epidemiol Biomakers Prev 1999;8(2):185-8.
18. Matthias C, Jahnke V, Jones PW, Hoban PR, Alldersea JE, Worrall Sf et al. Cyclin D1, Glutathione S-Transferase, and Cytochrome P450 genotypes and outcome in patients with upper aerodigestive tract cancers: assessment of the importance of individual genes using multivariate analysis. Cancer Epidemiol Biomakers Prev 1999;8(9):815-23.
19. Denissenko MF, Pao A, Tang M, Pfeifer GP. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science 1996;274(5286):430-2.
20. Sreelekla TT, Ramadas K, Pandey M, Thomas G, Nalinakumari KR, Pillai MR. Genetic polymorphism of CYP1A1, GSTM1 and GSTT1 genes in Indian oral cancer. Oral Oncol 2001;37(7): 593-8.
21. Drummond SN, de Marco L, Noronha JCM, Gomez RS. GSTM1 polymorphism and oral squamous cell carcinoma. Oral Oncol 2004;40:52-5.
22. Drummond SN, Gomez RS, Noronha JCM, Pordeus IA, Barbosa AA, de Marco L. Association between GSTT1 gene deletion and the susceptibility to oral squamous cell carcinoma in a cigarret-smoking subjects. Oral Oncol 2005;41:515-9.
23. Nair UJ, Nair J, Mathew B, Bartsch H. Glutathione S-transferase M1 and T1 null genotypes as risk factors for oral leukoplakia in ethnic betel quid/tobacco chewers. Carcinogenesis 1999;20(5):743-8.
24. Pemble S, Schroeder K, Spencer S, Meyer DJ, Hallier E, Bolt HM et al. Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem J 1994;300(1):271-6.
25. Rossit ARB, Conforti-Froes NDT. Suscetibilidade genética, biometabolismo e câncer. Sociedade Brasileira de Cancerologia 2000;10:22-30.
26. Bruhn C, Brockmoller J, Kerb R, Roots I, Borchert HH. Concordance between enzyme activity and genotype of glutathione S-transferases theta (GSTT1). Biochem Pharmacol 1998;56(9): 1189-93.
27. Seidgard J, Ekstrom G. The role of human glutathione transferases and epoxide hydrolases in the metabolism of xenobiotics. Environ Health Perspect 1997;105 Suppl 4:791-7.
28. Rebbeck TR. Molecular epidemiology of the human glutathione S-transferase genotypes GSTM1 and GSTT1 in cancer susceptibility. Cancer Epidemiol Biomarkers Prev 1997;6(9):733-43.
29. Abdel-Rahman SZ, Nouraldeen AM, Ahmed AE. Molecular interaction of 2,3-[14c]-acrylonitrile with DNA in gastric tissues of rat. J Biochem Toxicol 1994;9(4):121-8.
30. Abdel-Rahman SZ, El-Zein RA, Anwar NA. A Multiplex PCR procedure for polymorphic analysis of GSTM1 and GSTT1 genes in population studies. Cancer Lett 1996;107(2):229-33.
31. Ogden GR. Alcohol and oral cancer. Alcohol 2005;35:169-73.
32. Krauns KS, McClean MD, Nelson HH, Peters E, Calderon H, Kelsev KT. Duration but not intensity of alcohol and tobacco exposure predicts p16INK4A homozygous deletion in head and neck squamous cell carcinoma. Cancer Res 2006;66(8):4512-5.
33. Mc Mahon S, Chen AY. Head and neck cancer. Cancer Metastasis Rev 2003;22(1):21-4.
34. Wight AJ, Ogden GR. Possible mechanisms by which alcohol may influence the development of oral cancer-a review. Oral Onc 1998;34(6):441-7.
35. Carvalho AL, Singh B, Spiro RH, Kowalski LP, Shah JP. Cancer of the oral cavity: a comparison between institutions in a developing and a developed nation. Head Neck 2004;26(1):31-8.
36. Dobrossy L. Epidemiology of head and neck cancer: magnitude of the problem. Cancer Metastasis Rev 2005;24:9-17.
37. Rossit ARB, Cabral IR, Conforti-Froes NDT. Avaliação das freqüências alélicas de genes do biometabolismo em uma população brasileira. Genet Mol Biol 1999;22:23-23.
38. Rossini A, Rapozo DCM, Amorim LMF, Macedo JMB, Medina R, Neto JFM et al. Frequencies of GSTM1, GSTT1 and GSTP1 polymorphisms in a Brazilian population. Genet Mol Res 2002;1(3):233-40.
39. Buch SC, Notani PN, Bhisey RA. Polymorphism at GSTM1, GSTM3 and GSTT1 gene loci and susceptibility to oral cancer in an indian population. Carcinogenesis 2002;23(5):803-7.
40. Kietthubthew S, Sriplung H, Au WW. Genetic and environmental interactions on oral cancer in Southern Thailand. Environ Mol Mutagen 2001;37(2):111-6.
41. Hong YJ, Lee JK, Lee GH, Hong SI. Influence of glutathione S-transferase M1 and T1 genotypes on larynx cancer risk among Korean smokers. Clin Chem Lab Med 2000;38(9):917-9.
42. McWilliams JE, Evans AJ, Beer TM, Andersen PE, Cohen JI, Everts EC et al. Genetic polymorphisms in head and neck cancer risk. Head Neck 2000;22(6):609-17.
43. Liu CJ, Chang CS, Lui MT, Dang CW, Shih YH, Chang KW. Association of GST genotypes with age of onset and lymph node metastasis in oral squamous cell carcinoma. J Oral Pathol Med 2005;34:473-7.
44. Hamel N, Karimi S, Hebert-Blouin MN, Brunet JS, Gilfix B, Ghadirian P et al. Increased risk of head and neck cancer in association with GSTT1 nullizygosity for individuals with low exposure to tobacco. Int J Cancer 2000;87(3):452-4.
45. Minnard GC, Spitz MR, Wu X, Hong WK, Etzel CJ. Evaluation of glutathione S-transferase polymorphism and mutagen sensitivity as risk factors for the development of second primary tumors in patients previously diagnosed with early-stage head and neck cancer. Cancer 2006;105(12):2636-44.
46. Oude Ophuis MB, Manni JJ, Peters WH. Glutathione S-transferase T1 null polymorphism and the risk for head and neck cancer. Acta Otolaryngol 2006;126(3)311-7.
47. Konig-Greger D, Riechelmann H, Wittich U, Gronau S. Genotype and phenotype of glutathione-S-transferase in patients with head and neck carcinoma. Otolaryngol Head Neck Surg 2004;130(6):718-25.
48. Sherratt PJ, Manson MM, Thomson AM, Hissink EA, Neal GE, van Bladeren PJ, et al. Increased bioactivation of dihaloalkanes in rat liver due to induction of class theta glutathione S-transferase T1-1. Biochem J 1998;335:619-30.
49. Landi S. Mammalian class theta GST and differential susceptibility to carcinogens: a review. Mutat Res 2000;463:247-83.
50. Li R, Boerwinkle E, Olshan AF, Chambless LE, Pankow JS, Tyroler HA, et al. Increased bioactivation of dihaloalkanes in rat liver due to induction of class theta glutathione S-transferase T1-1. Biochem J 1998;335:619-30.
51. Li R, Folsom AR, Sharrett AR, Couper D, Bray M, Tyroler HA. Interaction of the glutathione S-transferase genes and cigarrete smoking on risk of lower extremity arterial disease: the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis 2001;154:729-38.