Year: 2003 Vol. 69 Ed. 1 - (8º)
Artigo Original
Pages: 40 to 45
Otolaryngology surgery: management of elective surgery in patients with haemophilia and von Willebrand disease
Author(s):
Marise P. C. Marques,
Érica S. T. Leite
Keywords: haemophilia, von Willebrand, otolaryngology surgery.
Abstract:
A 10-year prospective research was realized in 20 patients with haemophilia or von Willebrand disease (vWD). They were submitted to a total of 25 elective otolaryngological surgical events. The average age of the patients was 23,75 years (2-62 years). The study group consisted of 14 haemophiliacs, 11 with severe haemophilia A (1 female), 1 female with 30% of VIII factor (VIIIF) level, 1 male with haemophilia B and 1 female with severe factor X deficiency; and 6 with vWD, 4 type 1 (3 females), 1 male type 2 A and 1 male type 3. Acquired immunodeficiency syndrome was present in 13 haemophilic patients. The mean duration of the surgical events was 1 hour and 37 minutes (15 min-12 hours). The coagulation defect was corrected with desmopressin (DDAVP), intermediate purity VIIIF concentrate 8Y, cryoprecipitated or not activated protrombinic complex (PPSB), according to factors levels and the severity of the surgery. Epsilon aminocaproic acid was used associated. In 1 severe haemophiliac A patient an excessive bleeding was observed in the second day of the postoperative period which ceased with elevation of the minimal level of VIIIF to 80%. In another patient, with type 3 vWD, severe postoperative bleeding occurred, because of a difficult to identify the best reposition blood coagulation factor for him. After the use of intermediate purity VIIIF concentrate 8Y the bleeding was controlled. The haemostatic effect in the other patients was rated as normal or excellent. It is concluded that patients with vWD or haemophilia do not carry an increased operative risk if appropriate therapy is given.
![]()
INTRODUCTION
Hemophilia A and B are hereditary disorders of coagulation that result in defects of synthesis of factors VIII (FVIII) and IX (FIX), respectively. Both are transmitted as x-linked recessive disorders, practically only in men. The reduction of functional levels of FVIII and FIX results in prolongation of bleeding time. Hemophilia has always the same clinical presentation and specific dosage of factors is the only way to distinguish between them. The differentiation is important, since treatment is specific depending on the factor to be replaced1.
Depending on the levels of factors in the plasma, hemophilia can be classified in mild (6-25% of factor activity), moderate (1-5% activity), and severe (<1%). All members of a family with hemophilia have the same degree of factor deficiency1.
The von Willebrand disease (DvW) is the most frequent coagulation disorder, transmitted by dominant autosomal form. It is caused by a quantitative or qualitative abnormality of von Willebrand factor (FvW), which is a multimeric glycoprotein of high molecular weight, synthesized by endothelial cells and megakarocytes 2-5.
The main functions of FvW are: (a) to mediate the interaction between platelets and subendothelial collagen; (b) to mediate the platelet-platelet interaction; (c) to act as a molecular carrier of FVIII and stabilizer of the coagulating activity. The deficiency of FvW results in abnormalities in the primary and secondary phases of coagulation2, 4, 5.
The new classification of the International Society of Thrombosis and Hemostasis - ISHT identified 3 main types of DvW: (a) type 1 (about 80% of the cases) - quantitative partial deficiency of FvW; (b) type 2 - qualitative abnormality of FvW with 2 main subtypes: subtype 2 A - with absence of high molecular weight (HMW) multimers, and subtype 2 B - with increase in affinity of FvW to platelet glycoprotein 1b and secondary to loss of HMW multimers, usually followed by thrombocytopenia; (c) type 3 - severe deficiency of FvW with secondary deficiency of FVIII of variable degrees. The family pattern is not repetitive as in hemophilia, varying among the different family members4, 6, 7.
In the follow-up of patients with coagulation pathologies, it is important to treat acute hemorrhage and to provide peri-operative care and hemorrhage prophylaxis. Education is essential to these patients to reduce morbidity and mortality, especially concerning oral hygiene and hepatitis B and C vaccination. The use of intramuscular injection, as well as drugs such as non-steroidal antiinflammatories and others that inhibit platelet activity, should be avoided1, 2.
The patients with coagulopathies can be submitted to elective surgeries provided that there is a multidisciplinary professional team with excellent interactions between the surgeon, hematologist, hemotherapist and coagulation lab professional. It is important to correct coagulation during the surgical act and postoperatively, up to the scarring of the surgical wound1.
General recommendations include: (a) to exclude the presence of factor antibody inhibitor before the surgery; (b) to prevent the patients from taking anti-platelet medication before and after the surgery; (c) to perform the surgery in the beginning of the day and of the week to prevent administration problems, and (d) to be sure that there are enough replacement products for the peri-and postoperative periods, as well as reserved blood that has been phenotyped and submitted to transmissible disease screening for blood products1.
The purpose of this prospective study was to assess in a 10-year period the response to standardized treatment for correction of blood episodes in patients with hemophilia or von Willebrand disease who were submitted to small, middle and large ENT surgical procedures.
MATERIAL AND METHOD
We conducted a prospective study between March 1992 and February 2002 in 20 patients of both genders, aged from 2 to 62 years, with diagnosis of hemophilia or DvW and elective ENT surgical indication.
The patients followed a clinical protocol of peri-operative care, including study of hemostasis (platelet aggregation, factor dosage and inhibitor analysis), diagnosis and classification of coagulation disorder (type of hemophilia and severity: mild, moderate and severe, and type of DvW: type 1, type 2 and its subtypes, and type 3); serology to hepatitis and HIV; and correction of coagulation disorders peri-and post-operatively up to healing of the surgical wound with pharmacological agents: anti-fibrinolytic, vasopressin synthetic analog and/or blood products: replacement therapy according to the type and severity of coagulopathy, as well as the type of surgery.
The minimum factor level was elevated to 100% peri-operatively, considering that ENT surgeries have high hemorrhagic potential. We conducted daily minimum level dosage, maintained at 50% up to the 12th postoperative day.
We excluded from the study patients with plasma factor antibody inhibitor of coagulation.
We assessed clinical characteristics of the group of patients, the surgical procedure, the management employed, and efficiency of treatment to control peri- and postoperative bleeding.
RESULTS
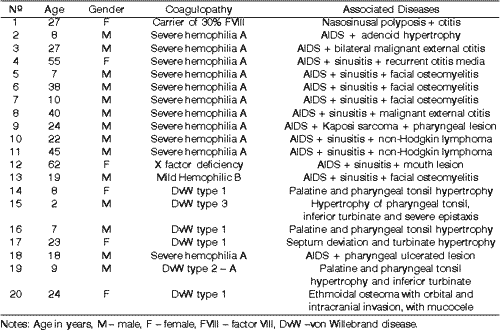
Of the 20 patients, 14 were male being 11 hemophilic (10 with severe hemophilia A and 1 with mild hemophilia B), and 3 had DvW. Of the female patients, 1 patient had the gene for hemophilia A, with 30% activity of FVIII, 1 was severe hemophilic A, with severe deficiency of factor X, and 3 had DvW.
Ages ranged from 2 to 62 years, mean age of 23.75 years. Out of the hemophilic patients, all had Acquired Immunodeficiency Syndrome (AIDS).
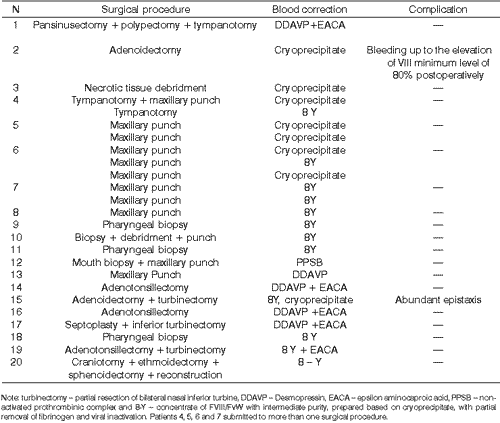
We conducted a total of 25 surgical procedures in the 20 patients. Patient 4 was submitted to 2 tympanotomies on different days, similarly to patients 5, 6 and 7, who underwent more than one maxillary punch through canine fossa on different times and with replacement after each procedure.
The procedures lasted on average 1 hour and 37 minutes, ranging from 15 minutes to 12 hours.
Patient 15, with type 3 DvW, had recurrent sinusitis with snoring and abundant preoperative epistaxis, submitted to various nasal packing procedures. He was submitted to treatment in order to try to better control sinusitis and epistaxis. We tested various types of FVIII/FvW concentrates, with good response only to 8-Y and cryoprecipitate. Right now, after a 6-year follow-up, he presents control of epistaxis and recurrent sinusitis episodes.
The patient 20, with type DvW, was operated together by the teams of Head and Neck Surgery and Neurosurgery. The surgery lasted 12 hours total, with peri- and post-operatively replacement of FVIII/FvW concentrate. There were no hemorrhagic complications.
The results were compiled in two tables emphasizing age, gender, coagulopathy, associated ENT diseases, surgical procedure conducted, correction of blood disorder, and postoperative complications (Tables 1 and 2).
Table 1. List of studied patients, including age, gender, coagulation pathology and associated ENT diseases.
Table 2. Correlation of studied patients, including the surgical procedure, postoperative complications and correction of blood disorders.
DISCUSSION
DvW was originally described in 1926 by the physician Erik von Willebrand. The disorder is related to mucous bleeding, including epistaxis, hypermenorrhea and excessive bleeding in surgeries and dental manipulations. In DvW, patients manifest qualitative or quantitative abnormality of FvW. There are more than 20 types and subtypes described. About 70% of the patients have type 1, 10-20% have type 2 A and 2 B and approximately 10% have type 3. Out of the group of patients with DvW in our study, there was higher prevalence of type 1 patients (4 patients), followed by type 2 A and type 3 (1 case each), agreeing with the literature. The clinical history of bleeding is important for the diagnosis. If DvW is suspected, we should conduct partial thromboplastin activated time (PTTa), bleeding time dosage, factor VIII:C, ristocetin co-factor, and von Willebrand antigen. Additional tests of platelet adhesion with ristocetin. and multimeric structure and tests of collagen band are important to differentiate the types of DvW. DvW affects about 1-3% of the population, and it is not diagnosed in most people, being twice more frequent than hemophilia. Even though the general prevalence of DvW is greater than that of hemophilia, in the present study the surgical patients most frequently had hemophilia (14 hemophilia : 6 DvW), probably due to the influence of the association with AIDS, which required interventions for the diagnosis and treatment of episodes cased by the viral disease2, 3, 4, 6, 8.
One of the main challenges in treating hemophilia is the development of antibody inhibitors of FVIII and FIX. The prevalence of FVIII inhibitors oscillates at about 4 to 20% in patients with hemophilia A. Only 1-4% of the patients with hemophilia B develop the inhibitors. The patients that present the greatest risk for developing inhibitors are those with severe hemophilia, patients younger than 30 years, with genetic predisposition, with antigenicity for factor replacement therapy and in groups of Black people, when compared to Caucasians. Patients with DvW type 3, poly-transfused, can also develop inhibitors, but it is rarer9-12.
The treatment of patients with coagulation factor inhibitors is important to interrupt acute hemorrhages, to improve hemostasis before and after surgeries and to induce immunotolerance to the factor. Elective surgeries are normally contraindicated in patients with inhibitor. For this reason, we excluded these patients from the elective ENT surgical protocol described here1, 9-11.
In the treatment of coagulopathy, the options are replacement transfusion therapy and non-transfusion therapy. The non-transfusion therapy recommends the use of desmopressin and anti-fibrinolytic agents12.
Desmopressin (1-deamino-8-Darginine vasopressin, DDAVP) is a synthetic analog of vasopressin that was originally developed for the treatment of diabetes insipidus and has been used in the treatment of DvW and hemophilia since 1977. DDAVP increased FVIII and FvW plasma concentrations, with no significant collateral effect, in healthy volunteers and in hemophilic patients with DvW. The action mode of DDAVP is not completely known yet. The addition of DDAVP to the culture of endothelial cells had no effect on the synthesis or secretion of FvW, and there is probably another route still not identified3, 13-14.
The advantages of DDAVP are low cost and no risk of transmitting diseases. It may be indicated for small surgeries and for small or middle sized bleeding episodes. In more severe bleeding episodes and large surgeries it is more appropriate to use FVIII/FvW concentrates1, 4, 12-14.
DDAVP is administered in the dosage 0.3 µg/Kg, diluted in 50 ml of saline solution and administrated intravenously for about 30 minutes. It increases 3 to 6 times the levels of FVIII and FvW in plasma in approximately 30 minutes, keeping them up for 6-8 hours. Infusions can be repeated every 12-24 hours, depending on the type and severity of bleeding. Subcutaneous access has also been used, at the dosage of 0.4µg/Kg. Intranasal DDAVP was developed for diabetes insipidus and night enuresis and not for hemophilia or DvW. Stimate® Nasal Spray is a vasopressin for intranasal use indicated in coagulopathies (150 µg/atomization) - 150 µg/day in patients weighting up to 50 Kg and 300 µg for patients with over 50 Kg of weight. Patients treated repeatedly with DDAVP can become less responsive to the drug1, 4, 12-14.
DDAVP is more effective in type 1 DvW, especially in those with normal platelets. The patients with mild and moderate hemophilia normally respond well. It is contraindicated in type 2 B DvW because it causes transient onset of thrombocytopenia. The patients with type 3 DvW or severe hemophilia do not respond to DDAVP. The agent was used in patients who had 30% FVIII, in patients with mild hemophilia and in patients with DvW type 1 in small and middle sized surgeries, and in four patients it was associated with anti-fibrinolytic agent, epsilon aminocaproic acid (EACA). In the patients in our study, bleeding control was considered excellent, agreeing with the data reported in the literature1, 11, 12, 14-16.
The activation and inhibition of hemostasis depend on the interaction of 4 biological systems: vascular walls, platelets, coagulation and fibrinolytic systems. The hemostatic mechanisms start in the vascular lesion site, forming a fibrin buffer. The coagulation process is regulated by physiological anti-coagulants, that is, the fibrinolytic system17.
Anti-fibrinolytic agents are synthetic drugs that inhibit the fibrinolysis, interfering in the plasminogen bound to fibrin, which prevents the lysis of a recently formed clot. EACA (50 mg/kg - QID) and tranexamic acid (25 mg/Kg - TID) are the most frequently used anti-fibrinolytic aminoacids. They can be administered by oral route, intravenously or topically, useful in cases of epistaxis, oral cavity and gastrointestinal bleedings, and metrorrhagia. They are useful in conditions of localized or generalized fibrinolysis increase. The hemostatic effect occurs by the fibrinolytic activity or by additional interaction with platelets and other unknown mechanisms. They are contraindicated in genital-urinary tract bleedings and in patients with potential risk for thrombosis1, 4, 5, 14, 18, 19.
Hormone therapy with estrogen can be used in order to elevate the plasma levels of FvW, but the response is variable and unpredictable, and it can not be fully used. In continuous clinical practice, they are very useful to reduce metrorrhagia severity in women with DvW, even in type 3, even though they do not modify the levels of FVIII-FvW. Hormone therapy was not used in any patients of the present study7.
In the replacement therapy of blood products, commercial concentrates for FVIII and FIX are better than cryoprecipitate or frozen fresh plasma, since they contain standard amounts of factor and have the lowest risk of viral disease transmission. The concentrates are prepared based on a pool of plasma derived from a large number of donors. A good selection of donors and the concentrated treatment with viral inactivation techniques reduce the risk of hepatitis and AIDS transmission. All patients that used blood products had severe hemophilia, that is, were not responsive to DDAVP 1.
The amount of FVIII to be infused depends on the therapeutic indication and the level of patients' factor. Postoperative follow-up of these patients should be conducted using minimum level of FVIII every 24 hours, as we did in the studied patients. In surgical patients with high hemorrhagic potential (such as ENT surgeries), we should elevate the minimum level to 100% for peri-operative conditions and keep the minimum postoperative level of 50% up to the 12th day. A severe hemophilic A patient presented postoperative bleeding with minimum level of 50% and after its elevation to 80%, there was suppression of bleeding. Each International unit (UI) infused by kg of weight (Kg) increases it in 2% (0.02 UI/ml), with an approximate half-life of 10-12 hours, as represented in the formula below:
FVIII dose (UI/Kg) = increase % of target FVIII X 0.5 1
Transfusion therapy with blood products that contain FVIII/FvW are the treatment of choice for patients with DvW that do not respond to DDAVP, types 2 and 3. We can use fresh frozen plasma (requiring large volumes), cryoprecipitate (5-10 x more FvW than fresh frozen plasma), or concentrates of intermediate purity and inactivated for viruses, such as 8Y. In the studied patients, we used 8Y in a type 1 patient instead of DDAVP owing to long surgical time, intensity of transoperative blood loss and extension of tumor disease, requiring partial resection of dura mater and its reconstruction and the anterior skull base, and in the 2-year-old child with type 3 disease6, 13, 16, 20, 21.
In a patient with severe deficiency of factor X, we used the prothrombinic complex (PPSB). PPSB is a plasma product that contains factors II, VII, IX and X, and proteins C and S. It is used in the treatment of hemophilia B replacement and in other deficiencies. It is also necessary to know the site and severity of bleeding, as well as plasma levels of factors and replacement of PPSB. The half-life of PPSB is 18-24 hours. The following formula is calculated:
Dose FIX (UI/Kg) = increase % of target FIX X 1.2 1
The activated coagulation factor shows a risk of producing thrombosis, with disseminated intravascular coagulation. The association with anti-fibrinolytic agents should be avoided because of the maximization of effects1.
In patients submitted to tonsillectomy, tonsilar sites were completely closed, preventing the presence of dead space and reduction of the exposed blood area. The patients undergoing turbinectomy and adenoidectomy were maintained with packing for 48 hours. Laser in the treatment of hemophilia patients is a very important support method because it reduces the risk of bleeding, such as myringotomy with OtoScan LASER and tonsillectomy with Nd YAG LASER, for dissection of tonsilar sites. High energy CO2 LASER has excellent hemostatic effect, especially in patients or lesions with increased potential of bleeding, mainly when compared to results of conventional procedures. We did not use the LASER in our patients because the device was not available3, 14, 19, 22-24.
Some authors stated that coagulation abnormalities were an absolute contraindication for surgery. In our study with 20 patients undergoing different types of surgeries, all of them had satisfactory progression, with no major complications and with the benefits of the surgery conducted. This observation has also been made by other authors, confirming that patients with hemophilia or DvW who are appropriately treated present no increased operative risks.3,15,25-27
CONCLUSION
Patients with different levels of hemophilia, as well as patients with von Willebrand disease, can safely undergo elective ENT surgeries when appropriately corrected for blood disorders. It is essential that the teams of ENT and hemotherapists have a good interaction.
REFERENCES
1. US Pharmacopeial Convention. Hemophilia Management. Transf Med Ver 1998;12(2):128-40.
2. Chang AC, Rick ME, Pierce LR, Weinstein LR. Summary of a workshop on potency and dosage of von Willebrand factor concentrates. Haemophilia 1998;4 (suppl.3):1-6.
3. Fehlberg GOD, Palheta-Neto FX, Palheta ACP, Melo MH, Gomes AP, Victorino RCC. Tonsilectomia em paciente com doença de von Willebrand. Rev Soc Otorrinol R J 2002;2(2):61-3.
4. Federici AB. Diagnosis of von Willebrand disease. Haemophilia 1998;4:645-60.
5. Ong YL, Hull DR, Mayne EE. Menorrhagia in von Willebrand disease successfully treated with single daily dose tranexamic acid. Haemophilia 1998;4:63-5.
6. Dobrkovska A, Krzensk U, Chediak JR. Pharmacokinetics, efficacy and safety of Humate-P ® in von Willebrand disease. Haemophilia 1998;4 (suppl.3):33-39.
7. Kouides PA. Females with von Willebrand disease: 72 years as the silent majority. Haemophilia 1998;4:665-76.
8. Kurth AA, Ludwig G, Scharrer I. Prevalence, pathophysiology, diagnosis and treatment of von Willebrand syndrome in orthopedic trauma patients. Orthopade 1999;28(4):366-74.
9. Manno CS. Treatment options for bleeding episodes in patients undergoing immune tolerance therapy. Haemophilia 1999;5(suppl.3):33-41.
10. Penner JA. Management of haemophilia in patients with high-titre inhibitors: focus on the evolution of activated prothrombin complex concentrate Autoplex ®T. Haemophilia 1999;5(suppl 3):1-9.
11. Shapiro A. Inhibitor treatment: state of the art. Seminars Hematol 2001;38(4):26-34.
12. Mannucci PM. Treatment of von Willebrand disease. Haemophilia 1998;4:661-4.
13. Gáspár L, Szabó G. Significance of the haemostaticus effect of LASERs in oral surgery. Orv Hetil J 1989;130 (41):2207-10.
14. Nitu-Whalley IC, Griffioen A, Harrington C, Lee CA J. Retrospective review of the management of elective surgery with desmopressin and clotting factor concentrates in patients with von Willebrand disease. Am J Hematol 2001;66(4):280-4.
15. Lusher JM. Clinical guidelines for treating von Willebrand disease patients who are not candidates for DDAVP - a survey of european physicians. Haemophilia 1998;4 (suppl.3):11-4.
16. Guyuron B, Zarandy S, Tirgan A. von Willebrand's disease and plastic surgery. Ann Plast Surg 1994;32(4):351-5.
17. Sindet-Pedersen S. Haemostasis in oral surgery-the possible pathogenetic implications of oral fibrinolysis on bleeding. Experimental and clinical studies of the haemostatic balance in the oral cavity, with particular reference to patients with acquired and congenital defects of the coagulation system. Dan Med Bull 1991;38 (6):427-43.
18. Berliner S, Horowitz I, Martinowitz U, Brenner B, Seligsohn U. Dental surgery in patients with severe factor XI deficiency without plasma replacement. Blood Coagul Fibrinolysis 1992;3(4):465-8.
19. Shah SB, Lalwani AK, Koerper MA. Perioperative management of von Willebrand's disease in otolaryngologic surgery. Laryngosope 1998;108:32-6.
20. Brown SA, Dasani H, Collins PW. Long-term follow-up of patients treated with intermediate FVIII concentrate BPL 8Y. Haemophilia 1998;4(suppl.3):89-93.
21. Metzner HJ, Hermentin P, Cuesta-Linker T, Langner S, Müller HG, Friedebold J. Characterization of factor VIII/von Willebrand factor concentrates using a modified method of von Willebrand factor mulimer analysis. Haemophilia 1998;4 (suppl.3):25-32.
22. Santos-Dias A. CO2 LASER surgery in hemophilia treatment. J Clin LASER Med Surg 1992;10(4):297-301.
23. Bent JP, April MM, Ward RF. Atypical indications for otoscan LASER-assisted myringotomy. Laryngoscope 2001;111(1):87-9.
24. Chellappah NK, Loh HS. LASER therapy for a haemophiliac. Case report. Aust Dent J 1990;35(2):121-4.
25. Betti ET & Lopes Filho O. Anginas - indicação cirúrgica de adenoidectomia e amigdalectomia. In: Tratado de otorrinolaringologia., 1ª Edição. São Paulo: Ed. Roca; 1994. p.169-179.
26. Hungria H. O problema das amígdalas e das vegetações adenóides. In: Otorrinolaringologia. 8ª Edição. Rio de Janeiro: Ed. Guanabara Koogan; 2000. p.165-170.
27. Sih T, Ramos BD, Sakano E, Endo L. Adenoamigdalectomia. In: Otorrinolaringologia. 1ª Edição. Rio de Janeiro: Ed. Revinter; 1998. p. 359-363.
1 Master in Otorhinolaryngology, UFRJ, physician with the Service of Otorhinolaryngology, HUCFF/UFRJ.
2 Physician, Service of Hemotherapy, HEMORIO /RJ.
Address correspondence to: Dra. Marise Marques Rua Santa Clara, 70/1101 - Copacabana Rio de Janeiro RJ 22041-010 - Tel/fax: (55 21) 2255.6365/2255.6014 - E-mail: marise@rionet.com.br
Affiliation: Service of Otorhinolaryngology, Hospital Universitário Clementino Fraga Filho (HUCFF)- Federal University of Rio de Janeiro (UFRJ) and Service of Hemotherapy, Instituto Estadual de Hematologia Arthur de Siqueira Cavalcanti do Rio de Janeiro (HEMORIO).
Head of the Service of Otorhinolaryngology, HUCFF/UFRJ: Prof. Dr. Shiro Tomita
Head of the Service of Hemotherapy HEMORIO: Dr. Luiz Amorim Filho.
Article submitted on September 12, 2002. Article accepted on November 29, 2002.