Year: 2005 Vol. 71 Ed. 5 - (1º)
Artigo Original
Pages: 554 to 558
Morphometric aspects of the human hypoglossal nerve in adults and elderly
Author(s): Romualdo Suzano Louzeiro Tiago1, Flávio Paulo de Faria2, Paulo Augusto de Lima Pontes3, Osíris de Oliveira Camponęs do Brasil4
Keywords: hypoglossal nerve, aging, dysphagia.
Abstract:
Aim: Perform a morphometric analysis of the myelinic fibers of the right hypoglossal nerve, in two age groups; with the purpose of verifying quantitative changes as a result of the aging process.
Study design: anatomic.
Material and Method: A 1cm fragment of the right hypoglossal nerve was collected from 12 male corpses without any medical history of diseases such as: diabetes, alcoholism, and malignant neoplasia. The sample was divided in two groups: group with six corpses under sixty years old (adult), and another group with six corpses sixty years old or above (elderly). The material was fixed in a 2.5% glutaraldehyde and 2% paraformaldehyde solution; postfixed in a 2% osmium tetroxide; dehydrated with increasing ethanol concentrations, and included in epoxy resin. Semi-thin sections of 0.3µm were obtaining, colored in 1% toluidine blue, and evaluated with a light microscope combined with a image analyzing system. The following morphometric data were quantified: intraperineural transversal section area, number, and diameter of the myelinic fibers.
Results: The intraperineural area of the hypoglossal nerve was similar on both age groups (p=0.8691). The average area on adult group was 1.697mm˛ and on elderly group was 1.649mm˛. The total number of myelinic fibers of the hypoglossal nerve was similar on both age groups (p=0.9018). The adult group presented an average of 10,286 ± 2,308 myelinic fibers, and the elderly group presented an average of 10,141 ± 1,590 myelinic fibers. A bimodal distribution of the myelinic fibers was observed, with a significant peak on the 9µm fibers, and another, smaller peak on the 2µm fibers. Conclusion: The intraperineural area and the total number of myelinic fibers of the right hypoglossal nerve is similar on both age groups.
![]()
INTRODUCTION
The aging process triggers modifications in the human body that are responsible for many different types of clinical manifestations, represented in the upper aerodigestive tract as vocal disorders and swallowing disorders. Oropharyngeal dysphagia is a frequent symptom in the elderly, especially in men aged over 60 years and it is normally associated with increase in duration of swallowing oropharyngeal phase.1
Many different authors have demonstrated that the aging process is also related with reduction of pharyngeal and supraglottic sensitivity 2, 3 and it is considered a factor responsible for the onset of dysphagia, aspiration and repetitive pneumonia in the elderly, owing to reduction of reflexes that protect the lower airways. Other modification observed in the elderly is the delay in opening of upper esophageal sphincter 1, 4, 5 and reduction of cricopharyngeal muscle tone 5.
Little has been studied about the action of aging in muscles 6, 7 and cranial nerves 8-12. A study carried out with the genioglossus muscle of rats demonstrated that there is reduction in number of neuromuscle joints in older rats. However, there was no difference in the nerve area responsible for innervation of the muscle 7. Research studies involving the laryngeal muscle system presented results that suggested numeric reduction of muscle fibers of thyroarytenoid muscle, especially slow contraction muscle fibers 13, reduction of proteins responsible for muscle contraction 14, and increase in connective tissue or endomise 15, 16. Other authors, based on electromyography assessment, presented results that suggested denervation of axonal lesion involving the motor control of larynx in the elderly, with consequent affection to contraction of laryngeal muscles 17. Morphometric studies of laryngeal nerves in humans demonstrated reduction of the number of myelin fibers, especially small diameter fibers.10,12
The purpose of the present study was to carry out morphometric analysis of the number and diameter of myelin fibers of the right hypoglossal nerve, in two age groups (adult and elderly), to check quantitative modifications resulting from the aging process.
METHOD
The research project was approved by the Research Ethics Committee, Hospital Sao Paulo/ Federal University of Sao Paulo (UNIFESP). We collected 1cm fragments of right hypoglossal nerve from 12 cadavers submitted to autopsy in the Service of Death Verification - University of Sao Paulo, between June 2003 and November 2004. Fragments were collected between 9 and 18 hours after the death and analyzed by the Center of Electron Microscopy, UNIFESP.
We selected male copses without past history of diabetes, alcohol abuse, malignant neoplasm or sudden weight loss 18-20. The sample was divided into two age groups: adult group (age below 60 years), comprising six corpses, and elderly group (age over 60 years), also comprising six corpses.
The fragment of right hypoglossal nerve was collected from the region after the crossing of the nerve with the internal and external carotid artery, after the emergence of the hypoglossal loop. The fragments were obtained by transversal section (perpendicular to the hypoglossal long axis) to enable quantification of the following morphometric data: area of transversal intraperineural section (area representing the number of myelin fibers), number and diameter of myelin fibers 21. Fragments were fixed in a solution at 2.5% glutaldehyde and 2% formaldehyde at buffer solution of sodium cacodilate 0.1 M, pH 7.4 (modified by Karnovsky, 1965),22 post-fixed in osmium tetroxide at 2% in buffer solution of sodium cacodilate 0.1M, pH 7.4, dehydrated in increasing concentrations of ethanol and included in resin type Araldite 502 .
The material was sectioned with ultramicrotome with glass knives to obtain super thin sections of 0.3 m thickness to be stained with toluidine blue at 1%. Sections were assessed under light microscope coupled to an image analyzer.
Morphometric assessment was divided into two steps:
A. Quantification of the total intraperineural area: to reach measurements of the area, images of the nerve were digitalized with a 5x objective that represented final magnification of 120x on the computer monitor screen. Intraperineural area was important for the calculation of the total number of fibers from the representative sampling.
B. Quantification of the number and external diameter of myelin fibers: images of the nerve were digitalized using a 40x objective, which represented total final enlargement of 1920x on the computer monitor screen. Four random fields were assessed per slide 23, and in addition to counting fibers and measuring the diameters, we measured the representative area of the field, which meant exclusion of the perineural areas when they appeared in the field. Thus, in each slide, we assessed the area that ranged from 7% to 14.2%. The total number of myelin fibers was estimated by the total intraperineural area (obtained in the first step) and the number of fibers and area of the assessed fields (obtained in the second step).
To prevent errors in sampling (margin effect) we excluded the myelin fibers projected over the lower and left lines that limit the field 24. The smaller diameter of the fiber (longer perpendicular distance to the longer axis of the myelin fiber) was chosen to measure myelin fibers with elliptical or irregular perimeter.10,21
To compare the means of intraperineural area and the number of myelin fibers among the adult and elderly groups, we used the statistical method of variance analysis (ANOVA). We adopted the level of significance of 0.05. p value (likelihood of occurrence of an event) < 0.05 was considered as significant and it determined rejection of the equality hypothesis between the groups.
RESULTS
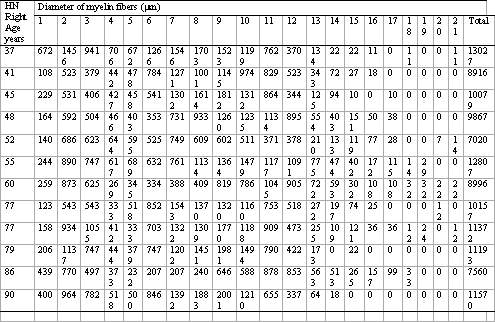
Individual data about the number of myelin fibers according to diameter are presented in Table 1. The mean age of the Adult group was 46.3 years and of the Elderly group was 78.2 years. To better visualize the morphometric analysis of the right hypoglossal nerve, we present Figures 1, 2 and 3. In Figure 2 we present the typical photomicrographies of the transversal section area (smaller magnification) and of the field (larger magnification) representing each age group.
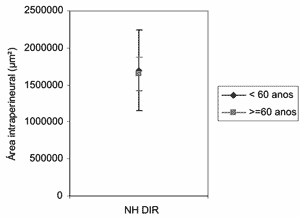
Figure 1. Profile of means of intraperineural area (µm2) of right hypoglossal nerve, according to age group.
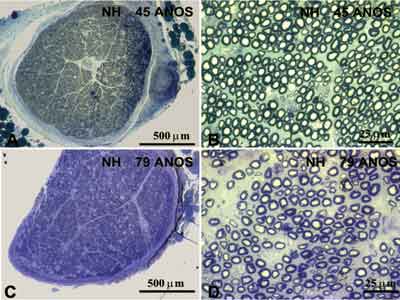
Figure 2. Typical photomicrography of right hypoglossal nerve of a subject aged 45 years (A and B) and a subject aged 79 years (C and D). In A and C we can observe the transversal section of the nerve, stained with toluidine blue. In B and D we can observe the field of a transversal section of the nerve, stained with toluidine blue.
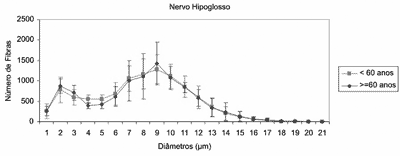
Figure 3. Profiles of mean number of myelin fibers of right hypoglossal nerve in the groups <60 years and > 60 years, according to fiber diameter.
Table 1. Individual data of number of myelin fibers of right hypoglossal nerve according to diameter of myelin fibers.
HN Right Age years Diameter of myelin fibers ( m)
Key: HN Right = Right hypoglossal nerve
In the intraperineural area of the right hypoglossal nerve (Figures 1 and 2) we did not observe any difference between the two age groups (p=0.8691). The mean of Adult group area was 1,696,918µm2 (1.697 mm2) and in the elderly group it was 1,649,083µm2 (1.649mm2).
In the analysis of right hypoglossal nerve (Figure 3) we did not detect difference between the two age groups concerning total number of myelin fibers (p=0.9018) and distribution of fibers according to the diameter. The adult group presented mean of 10,286 2,308 of myelin fibers and the elderly group presented mean of 10,141 1,590 of myelin fibers.
DISCUSSION
The hypoglossal nerves, form the cranial nerve, are responsible for motor innervation of tongue muscles. The body (pericardium) of lower motor neurons of hypoglossal nerve is located in the bulb, close to the midline and under the floor of the fourth ventricle. Axons of these neurons leave the hypoglossal nucleus as a row of small fascicles and form the hypoglossal nerve that goes down the neck; close to the hyoid bone it directs anteriorly and penetrates into the tongue body to innervate muscles hypoglossus, genioglossus, styloglossus and tongue intrinsic muscles.25
At the level of the hyoid, the hypoglossus nerve is normally comprised of one single fascicle.26-28 Our results demonstrated that the mean of intraperineural sectional area in the Adult group (1.697mm2) was similar to that of the elderly group (1.649mm2), with no statistically significant difference (Figures 1 and 2). Many different authors have studied the morphometric characteristics of the hypoglossus nerve to reach data that could support the technique of hypoglossus-facial anastomosis in the treatment of peripheral facial palsy. These authors described the mean area that ranged from 1.54 to 2.02mm2, close to the hyoid bone;27-29 however, the authors did not report whether the assessed area included epineurum or not and if it measured only the area that represented the nerve (intraperineural area).
The mean number of myelin fibers in the Adult group was 10,286 fibers and in the elderly group it was 10,141 fibers, without difference between the groups. In the two age groups we observed bimodal distribution (Figure 3), with peaks of fibers of 2 m and 9 m and predominance of medium diameter myelin fibers. As a result of aging, we did not observe reduction in number of myelin fibers of the hypoglossus nerve. Some studies assessed the total number of myelin fibers of the hypoglossus nerve, as well as the distribution of fibers according to the diameter and they described similar results as the ones we observed here. In those studies, the total number of fibers ranged from 9,202 to 9,920 fibers26,27,29, with bimodal distribution, predominance of 8-9mm fibers and smaller peak in 3mm fibers.27,29 Atsumi et al. (1987) suggested that this small peak of myelin fibers may represent afferent fibers or fibers of the autonomous nervous system that are integrated in the hypoglossus nerve after passing through the hypoglossus canal on the skull base.29
Despite the fact that we did not observe modification in total number and in distribution of myelin fibers between adult and elderly subjects, the literature reports that some diseases involve the lower motor neuron, such as amyotrophic lateral sclerosis, leading to reduction in number of medium diameter myelin fibers (5-10mm), which results in tongue atrophy; this is one of the main signs of advanced stage of the disease.29
Aging seems to determine a selective loss of myelin fibers, and it is quite common in elderly to have reduction of the number of smaller diameter fibers of laryngeal nerves that are responsible for sensitivity 10,12 and by the motor innervation of slow contraction muscle fibers.30 Schwann cells, from the myelin sheath, are responsible for nourishing the axon, and the thicker the myelin sheath, the more protected the axon. Given that the tongue muscle is comprised mainly by fast contraction muscle fibers 31,32, innervated by medium diameter myelin fibers (5-10 m), the reduction of these myelin fibers practically does not occur in the hypoglossus nerve of elderly patients.
CONCLUSION
Even though aging determines modifications of the oropharyngeal phase of swallowing, we did not observe morphometric abnormalities of the hypoglossal nerve on the right side in adult and elderly subjects. The intraperineural area, the total number and distribution of myelin fibers were similar in both age groups.
REFERENCES
1. Robbins J, Hamilton JW, Lof GL, Kempster GB. Oropharyngeal swallowing in normal adults of different ages. Gastroenterol 1992; 103:823-9.
2. Aviv JE, Martin JH, Jones ME, Wee TA, Diamond B, Keen MS, Blitzer A. Age-related changes in pharyngeal and supraglottic sensation. Ann Otol Rhinol Laryngol 1994; 103: 749-52.
3. Aviv JE. Effects of aging on sensitivity of the pharyngeal and supraglottic areas. Am J Med 1997; 103: 74s-76s.
4. Ekberg O, Feinberg MJ. Altered swallowing function in elderly patients without dysphagia: radiologic findings in 56 cases. AJR 1991; 156: 1181-4.
5. McKee GJ, Johnston BT, McBride GB, Primrose WJ. Does age or sex affect pharyngeal swallowing? Clin Otolaryngol 1998; 23: 100-6.
6. McComas AJ. Oro-facial muscles: internal structure, function and ageing. Gerondotology 1998; 15: 3-14.
7. Hodges SH, Anderson AL, Connor NP. Remodeling of neuromuscular junctions in aged rat genioglossus muscle. Ann Otol Rhinol Laryngol 2004; 113: 175-9.
8. Malmgren LT, Ringwood MA. Aging of the recurrent laryngeal nerve: an ultrastructural morphometric study. In: Fujimura O, editor. Vocal physiology: voice production, mechanisms and functions. New York: Raven Press; 1988. p. 159-80.
9. Rosenberg SI, Malmgren LT, Woo P. Age-related changes in the internal branch of the rat superior laryngeal nerve. Arch Otolaryngol Head Neck Surg 1989; 115: 78-86.
10. Mortelliti AJ, Malmgren LT, Gacek RR. Ultrastructural changes with age in the human superior laryngeal nerve. Arch Otolaryngol Head Neck Surg 1990; 116: 1062-9.
11. Nakai T, Goto N, Moriyama H, Shiraishi N, Nonaka N. The human recurrent laryngeal nerve during the aging process. Okajimas Folia Anat Jpn 2000; 76: 363-8.
12. Tiago RSL, Munhoz MSL, Faria FP, Guilherme A. Aspectos histomorfométricos do nervo laríngeo superior. Rev Bras Otorrinolaringol 2002; 68: 157-65.
13. Malmgren LT, Fisher PJ, Bookman LM, Uno T. Age-related changes in muscle fiber types in the human thyroarytenoid muscle: an immunohistochemical and stereological study using confocal laser scanning microscopy. Otolaryngol Head Neck Surg 1999; 121: 441-51.
14. Suzuki T, Connor NP, Lee K, Bless DM, Ford CN, Inagi K. Age-related alterations in myosin heavy chain isoforms in rat intrinsic laryngeal muscles. Ann Otol Rhinol Laryngol 2002; 111: 962-7.
15. Rodeńo MT, Sánchez-Fernández JM, Rivera-Pomar JM. Histochemical and morphometrical age in changes in human vocal cord muscles. Acta Otolaryngol 1993; 113: 445-9.
16. Kersing W, Jennekens FGI. Age-related changes in human thyroarytenoid muscles: a histological and histochemical study. Eur Arch Otorhinolaryngol 2004; 261: 386-92.
17. Takeda N, Thomas GR, Ludlow CL. Aging effects on motor units in the human thyroarytenoid muscle. Laryngoscope 2000; 110: 1018-25.
18. Shuman CR, Weissman B. Recurrent laryngeal nerve involvement as a manifestation of diabetic neuropathy. Diabetes 1968; 17: 302.
19. Dyck PJ. Hypoxic neuropathy: does hypoxia play a role in diabetic neuropathy? Neurology 1989; 39: 111-8.
20. Grisold W, Drlicek M. Paraneoplastic neuropathy. Curr Opin Neurol 1999; 12: 617-25.
21. Fraher JP. On methods of measuring nerve fibres. J Anat 1980; 130: 139-51.
22. Karnovsky MJ. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 1965; 27: 137A-8A.
23. Mayhew TM, Sharma AK. Sampling schemes for estimating nerve fibre size: methods for nerve trunks of mixed fascicularity. J Anat 1984; 139: 45-58.
24. Gundersen HJG. Notes on the estimation of the numerical density of arbitrary profiles: the edge effect. J Microsc 1977; 111: 219-23.
25. Burt AM. Neuroanatomia. Rio de Janeiro: Guanabara Koogan; 1995. p. 343-4.
26. Mackinnon SE, Dellon AL. Fascicular patterns of the hypoglossal nerve. J Reconstr Microsurg 1995; 11: 195-8.
27. Asaoka K, Sawamura Y, Nagashima M, Fukushima T. Surgical anatomy for direct hypoglossal-facial nerve side-to-end "anastomosis". J Neurosurg 1999; 91: 268-75.
28. Vacher C, Dauge MC. Morphometric study of the cervical course of the hypoglossal nerve and its application to hypoglossal facial anastomosis. Surg Radiol Anat 2004; 26: 86-90.
29. Atsumi T, Miyatake T. Morphometry of the degenerative process in the hypoglossal nerves in amyotrophic lateral sclerosis. Acta Neuropathol 1987; 73: 25-31.
30. Sasaki CT, Kim Y, Sims HS, Czibulka A. Motor innervation of the human cricopharyngeus muscle. Ann Otol Rhinol Laryngol 1999; 108: 1132-9.
31. Sutlive TG, Shall MS, McClung JR, Goldberg SJ. Contractile properties of the tongue's genioglossus muscle and motor units in the rat. Muscle Nerve 2000; 23: 416-25.
32. Saigusa H, Niimi S, Yamashita K, Gotoh T, Kumada M. Morphological and histochemical studies of the genioglossus muscle. Ann Otol Rhinol Laryngol 2001; 110: 779-84.
Ph.D. in Sciences, Program of Post-graduation in Otorhinolaryngology and Head and Neck Surgery, UNIFESP. Physician, Researcher, Department of Otorhinolaryngology and Head and Neck Surgery, Federal University of Sao Paulo - Escola Paulista de Medicina.
Ph.D. in Sciences, University of Sao Paulo, Joint Professor, Center of Electron Microscopy, Federal University of Sao Paulo - Escola Paulista de Medicina.
Full Professor, Department of Otorhinolaryngology and Head and Neck Surgery, Federal University of Sao Paulo - Escola Paulista de Medicina. Faculty Professor, Department of Otorhinolaryngology and Head and Neck Surgery, Federal University of Sao Paulo - Escola Paulista de Medicina.
Ph.D. in Medicine, Federal University of Sao Paulo - Escola Paulista de Medicina. Professor, Post-graduation Program in Otorhinolaryngology and Head and Neck Surgery, Federal University of Sao Paulo - Escola Paulista de Medicina.
Address correspondence to: Romualdo Suzano Louzeiro Tiago - Rua Pio XII 439 ap. 122 Bela Vista Sao Paulo SP 01322-030.
Tel. (55 11) 3285-6824 - E-mail: romualdotiago@uol.com.br
The present article was submitted by SGP on 23/Apr/2005 and approved on 19/Sept/2005.