Year: 2002 Vol. 68 Ed. 1 - (2º)
Artigo Original
Pages: 07 to 13
Otoprotection of auditory hair cells against amikacin ototoxicity
Author(s):
José Antonio A. de Oliveira 1
Daniel Mendes Canedo 2,
Maria Rossato 3
Keywords: ototoxicity, aminoglycoside antibiotics, amikacin, self-protection.
Abstract:
The objective of the present study was to determine the occurrence of self-protection of hair cells against the ototoxicity of amikacin in guinea pigs. Study design: experimental. Material and method: The outer hair cells of the organ of Corti of 31 animals were studied by scanning electron microscopy. The animals were divided into four groups: A, control; B, amikacin, 20 mg/kg/day for 30 days; C, amikacin, 400 mg/kg/day for 12 days; group D, amikacin, 20 mg/kg/day for 30 days followed by 400 mg/kg/day for 12 days. Results: In groups A and B, the cells were normal in all cochleae, in group C there were extensive lesions of the two more basal turns, in group D there was a significant reduction of lesions in the two more basal turns compared to group C, which had received the ototoxic dose of amikacin alone. Conclusion: We concluded that the non-ototoxic dose of amikacin administered before the ototoxic dose of the same antibiotic had a statistically significant protective effect on the two more basal turns of the guinea pig cochlea.
![]()
1 Faculty Professor, Head of the Clinic.
2 Post-graduation studies under course, Area of Otorhinolaryngology
3 Technician of the Service of Otorhinolaryngology.
Service of Otorhinolaryngology, Hospital das Clínicas, Medical School of Ribeirão Preto, University of São Paulo.
Supported by FAPESP and CNPq.
Presented as "Contributed Paper" 2000 Annual Meeting Of American Academy Of Otolaryngology Head Neck Surgery".
Address correspondence to: Serviço de Otorrinolaringologia do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo.
Av. Bandeirantes, 3900 CEP 14049-900 - Ribeirão Preto - Tel: (55 16) 602 2862 - Fax (55 16) 602 2860 - Home: (55 16) 623 2878.
Article submitted on September 13, 2001. Article accepted on October 29, 2001.
INTRODUCTION
The organ of Corti is the fundamental neuro-sensorial structure of peripheral auditory processing of acoustic message. It is formed by a series of complex epithelial cells, with sensorial and support cells, located on the basilar membrane. Sensorial cells are of two types: outer hair cells and inner hair cells.
Different pharmacological groups have ototoxic potential and aminoglycoside antibiotics are the most frequently studied ones. Transient and permanent auditory function and vestibular disorders induced by therapeutic nature aminoglycoside comprise the ototoxicity.
Aminocyclitolic aminoglycoside antibiotics are undoubtedly the most important ones because they are effective and cheap and one of the most commonly used drugs nowadays around the world, especially in developing countries. They form a family of antibiotics that have a complex chemical structure, are active and used mainly against gram-negative bacilli and in tuberculosis, with bactericidal action against sensitive germs. Owing to its polycathionic nature (pH between 7 and 8), aminoglycoside molecules interact strongly with polyphosphoinositides present in the hair cells of the inner ear7. Complexes of aminoglycoside antibiotics and polyphosphoinositides produce a number of inhibiting factors in the receptors of cellular membrane, blocking the calcium channel and leading to Corti's organ damage. Lesions are mainly located in the outer hair cells4. The mechanism of chronic toxicity of aminoglycoside has been recently understood thanks to a series of in vivo and in vitro studies14. The main finding was that gentamicin is capable of chelating iron, forming a compound with oxidative capabilities22. The complexes may be formed also by other aminoglycoside17 and they cause formation of free radicals that can oxide a great variety of substances, such as proteins, membrane lipids, DNA. These free radicals are biological agents that cause tissue damage7. The complex seems to be the toxic metabolite that results in ototoxic damage of hair cells, especially the outer hair cells of Corti's organ, by the formation of free radicals.
Streptomycin was the first drug from the group of aminoglycoside initially used to treat tuberculosis at the end of the Second World War. Since then, ototoxic and vestibulotoxic effects are known. As time went by, other aminoglycosides were introduced in the market, but the ones currently in use are amikacin, gentamicin and tobramicin. All these aminoglycosides have an amina group that guarantees the basic characteristics responsible for its bactericidal anti-infectious action over the gram-negative bacteria, but also its toxic effects over the inner ear and the kidney15. Despite the appearance of other drugs that also fight against gram-negative bacteria, such as quinolone and 3rd generation cephalosporins, aminoglycoside are still used all over the world owing to its efficacy, wide activity spectrum and low cost.
Amikacin is a semi-synthetic aminoglycoside derived from kanamicin, active against most microbial species resistant to gentamicin and kanamicin 1. Many clinical studies on the effects of amikacin on humans have revealed that it may cause alterations such as tinnitus, high frequency hearing loss and, more rarely, vestibular disorders18. One of the most serious limitations in the clinical application of the drug is induced ototoxicity, which causes permanent damage to the cochlear and vestibular systems6. Amikacin at a dose of 400mg/Kg/day IM causes total elimination of outer hair cells and partial damage to the inner hair cells, on the first and second turn of the cochlea of guinea pigs, plus less significant damage on the 3rd and 4th turns, followed by partial damage to inner hair cells8. Considering the three rows of outer hair cells, the damage initially affects the first row, progressing to the second and third rows, on the most apical turns12. Scanning electron microscopy, a method widely used in ototoxicity studies, enables a careful examination of normal and abnormal Corti's organ at the level of the cochlea8.
The phenomena of otoprotection against traumatic noise have recently been studied: they are called conditioning and toughening, developed by the cochlear hair cells. Sound conditioning consists of the exposure of the animal to a low-level acoustic continuous stimulus, not harmful to hearing3. The phenomenon called toughening is caused by the exposure to interrupted non-harmful acoustic stimuli. These low intensity non-harmful stimuli, applied for a long time, before exposure to a traumatic type of noise, protect the cochlea of animal models against traumatic noise, reducing the physiological alterations and damage of cochlear hair cells3. Hair cells would be modified by the conditioning stimuli and protection seems to be measured by alterations limited to the cochlea23.
We devised a otoprotection study against ototoxicity using the aminoglycoside amikacin. The technique used was scanning electron microscopy to study structural lesions of Corti's organ, especially outer hair cells, in a group of guinea pigs, which received different doses of amikacin aminoglycoside antibiotic, assessing the extension of damage to each cochlear turn. The study intended to confirm whether previous prolonged administration of non-harmful amikacin aminoglycoside doses, far lower than ototoxic doses, would protect the Corti's organ against damage caused by posterior application of the same amikacin at ototoxic doses.
MATERIAL and Method
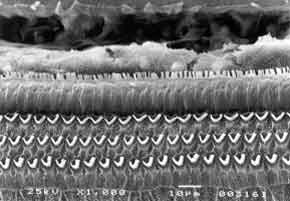
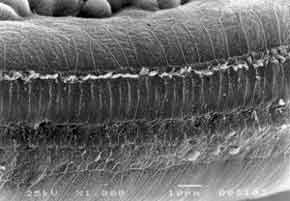
In the present experiment, we used 31 albino male guinea pigs, weighting from 250 to 280g, with normal Preyer reflex. The animals remained in the Laboratory of Experimental Surgical Technique, Medical School of Ribeirão Preto, University of São Paulo (FMRP-USP). The animals were used according to the guidelines of the institution which is based on care for use of laboratory animals, by the Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, National Academy Press, Washington, D.C., 1996. The drug used by the present study was amikacin aminoglycoside administered intramuscularly (IM). Preyer reflex was tested daily, together with weight measuring, in order to control the sacrifice day of each animal. Guinea pigs were divided into 4 groups: Group A - control group (placebo), consisted of 5 guinea pigs (10 cochleae) that received IM distilled water for a 30 day period and were then sacrificed; group B - formed by 5 guinea pigs (10 cochleae) that received IM amikacin 20 mg/kg/day for 30 days and then were sacrificed; group C, consisted of 7 guinea pigs that received IM amikacin 400 mg/kg/day, up to the moment Preyer reflex was absent (13 cochleae, one was discarded because of technical problem); and group D, consisted of 14 guinea pigs that received IM amikacin 20 mg/kg/day, for 30 days and then 400 mg/kg/day for the mean period during which there was absence of Preyer reflex in the group, that is, 12 days (26 cochleae), two cochleae were discarded because of technical problems. After decapitation, temporal bones that contained the bulla were removed. Bullae were opened and on the apex and on the cochlear round window, in which we injected glutaldehyde at 2.5% and phosphate buffer 0.1M (Sorensen), at 4o C for fixation. Microdissection was made so that to preserve the turns. The material was preserved for 12 hours in buffered solution at 0.1M, and then re-fixed with osmium tetroxide at 1% and phosphate buffer at 0.1M for one hour at 4o C. Dehydration was made using ethanol. Drying was performed by the critical point method, using liquid carbon dioxide with the device BALTEC-CPD 030 - "CRITICAL POINT DRYER". The cochlea was placed in a specimen holder cylinder with carbon conducting paste. We then proceeded with metalization of the cochlea so that it could be perfectly visualized under scanning electron microscopy (JEOL JMS - 5200), which is done by inserting a thin layer of gold, using the vaporizer (BAL-TEC SDC 050). Result analysis was conducted next. Guinea pigs' turns were photographed and assessed. The most apical portion of the cochlea that had the smallest size was turn 4. Representative samples of each turn with 150 outer hair cells in three rows and 50 inner hair cells were used in the study. Numbers and percentages of cochleae with normal cells (N), completely damaged (TD), partially damaged (PD) cells were calculated for each turn of each group. We assessed the cochleae of each group of the four studied groups (two cochleae per guinea pig). The values were compiled in a Table and analyzed with the non-parametric chi-square test to investigate significant result differences among the groups. Percentage values were plotted in graphs to compare the groups.Figure 1. Guinea pig of group A. Microstructure of Corti's organ with normal outer hair cells (OHC); inner hair cells (IHC) and support cells (SC). Scanning Electron Microscopy. Magnification 1000X.Figure 2. Guinea pig of group C. Microstructure of Corti's organ. Greater extension of damage on basal turn 1, outer hair cells (OHC). Scanning Electron Microscopy. Magnification 1000X.Figure 3. Guinea pig of group D. Microstructure of Corti's organ with large quantity of normal outer hair cells (OHC). Inner hair cells (IHC) are normal or have minor damage. Scanning Electron Microscopy. Magnification 500X.Graph 1. Percentage of cochleae with normal cells in turns of guinea pigs of group C (treated with 400mg Amikacin) and group D (treated with 20mg + 400 mg Amikacin).Graph 3. Percentage of cochleae with partially damaged cells, in turns of guinea pigs of group C (treated with 400 mg Amikacin) and group D (treated with 20mg + 400 mg Amikacin).
RESULTS
GROUP A - Control (normal guinea pigs) - All guinea pigs presented normal outer hair cells in all turns of the cochlea (Figure 1). GROUP B - All guinea pigs presented Preyer reflex present when sacrificed. Corti's organ and hair cells were normal in all turns of the cochlea. GROUP C - We detected damage to outer hair cells in all turns, in most cochleae. Basal turns (T1 and T2) presented high degree of damage and outer hair cells had practically disappeared (Figure 2). Inner hair cells were present in some small areas of damage. Turns T3 and T4 (more apical) presented less extensive damage. GROUP D - In basal turns T1 and T2 we observed a great amount of normal outer hair cells and some more significant partial damage to the first row of outer hair cells. Inner hair cells were normal (Figure 3). In the more apical turns (T3 and T4) there was predominance of damage to the first row of outer hair cells, occasional damage in the second row and few lesions in the third row. Inner hair cells had few lesions.
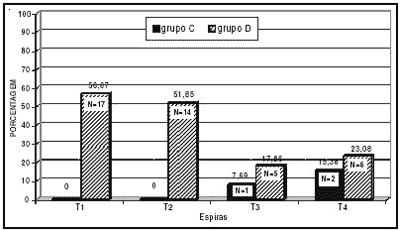
Graph 1 shows the percentage and number of cochleae with normal outer hair cells (N) in the different guinea pigs turns of group C. We can observe that in turns T1 and T2 we did not find normal cells, but only a small amount of normal cells in turns T3 and T4. We could also observe that from the most basal to the most apical there was a slight increase in percentage of cochleae with normal cells, showing that the most basal turns (T1 and T2) of the cochleae are more sensitive to the ototoxic agent, decreasing in sensitivity towards the apex. The Graph shows also the percentage of cochleae with normal outer hair cells (N) in the different cochlear turns of group D guinea pigs. We could observe a percentage of 56.67 in turn T1, 51.85 in turn T2, 17.86 in turn T3 and 23.08 in turn T4. The percentage of normal outer hair cells (N) present was higher in basal turns (T1 and T2), decreasing in the apical ones (T3 and T4). The Graph shows the differences between percentage of cochleae with normal outer hair cells in turns T1 and T2 in groups C and D. Based on the analysis of the number of cochleae with normal cells in groups C and D by the statistical chi square test, the differences observed between turns T1 and T2 of groups C and D were statistically significant, which did not happen for turns T3 and T4. These data show the effective protection of outer hair cells on the basal turns (T1 and T2) in group D, which received a non-ototoxic dose (20 mg/kg/day) during 30 days before the toxic dose of 400 mg/kg/day was administered.
In graph 2, we can see the percentages of cochleae with outer hair cells completely damaged (TD), in different guinea pigs of group C. The graph shows the values of turn T1 (100%), turn T2 (72.73%), turn T3 (53.85%) and turn T4 (30.77%). The percentage of cochleae with outer hair cells completely damaged (TD) in this group is higher in the first and second turns (T1 and T2) decreasing gradually up to the fourth turn (T4), showing the high toxicity of the drug, at that dose, to basal turns. In more apical turns (T3 and T4), the number of cochleae with outer hair cells completely destroyed is smaller. The graph shows the percentage of cochleae with outer hair cells completely damaged (TD) in the cochlear turns of group D guinea pigs, which were treated with 20mg/Kg/day dose amikacin before receiving the toxic dose of 400 mg/kg/day. As we can see, values are much lower for group D concerning basal outer hair cells: T1, 33.33%; and T2, 22.22%; T3, 25%; T4, 15.38%. Differences were significant for the two basal turns (T1 and T2) and not significant in the apical turns (T3 and T4). The Graph allows comparison of percentage of cochleae completely destroyed (TD) cells in turns of guinea pigs of groups C and D. It may be observed that in all turns, the percentage of cochleae with outer hair cells completely destroyed was higher for group C than for group D. The difference found between number of completely damaged cochleae in turns 1 and 2 of groups C and D was statistically significant according to the chi-square test.
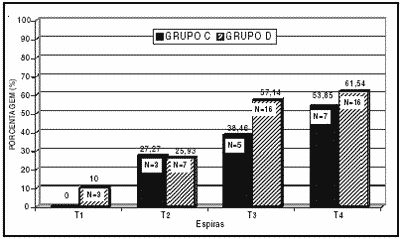
Graph 3 shows the percentage of cochleae with outer hair cells partially damaged in the different turns of group C guinea pigs. The results (turn T1, 0%; turn T2, 27.27%; turn T3, 38.46% and turn T4, 53.85%) showed gradual increase in the percentage of partially damaged outer hair cells (PD) in the basal turns compared to the apical ones. The graph shows the percentage of cochleae with partially damaged cells (PD) in the turns of the cochleae of group D guinea pigs; we observed the small percentages of partially damaged outer hair cells in turns T1 and T2, and damage increased towards the most apical turns T3 and T4. The difference between percentage of cochleae with partially damaged outer hair cells in the different turns of group C and D is small, but in both groups it increases from the basis to the apex. In such cases, statistical studies with chi-square test did not show statistically significant difference between the groups.
DISCUSSION
Experimental studies have been performed to find less toxic therapies and more effective prevention means to protect the cochlea against chemical and physical agents. The finding about conditioning and toughening phenomena to traumatic noise by previous exposure to low intensity noise encouraged us to conduct a trial in order to assess the cochlea, Corti's organ as a whole and the outer hair cells that could develop some mechanism of otoprotection against aminoglycoside ototoxicity. The mechanisms that could determine otoprotection against noise are unknown, but their presence in different animal species suggest the involvement of anatomical, physiological, metabolic and homeostatic processes in the auditory system of mammals, activated by stress of conditioning noise11.
In studies with amikacin, we assessed the different cochlear turns in guinea pigs of groups A and B and there were no alterations of Corti's organ. All preparations presented outer hair cells in the 3 rows, support cells with no modification to their structure and normal inner hair cells. In Group C, all turns were very degenerated with major structural alterations in outer hair cells. Other authors also found high levels of ototoxicity at the same doses13. When we compared groups C (400 mg/kg/day) and D (20 mg/kg/day for 30 days and after 400 mg/kg/day), we noticed a marked difference concerning the presence of normal outer hair cells in cochleae of guinea pigs. There was preservation of hair cells, with normal aspect in many areas, especially in the two basal turns, showing a protective effect that was statistically significant using a previous dose of 20mg/Kg/day por 30 days before the ototoxic dose, when lesions were compared to the result of the application of toxic dose of amikacin only. We could observe that the most significant protection occurred in the basal turns of the group, the site where there was high sensitivity to lesions in group C. It made us consider that hair cells can develop a protection mechanism during the application of the non-ototoxic stimulus that enabled the later resistance to the harmful dose. There was evident and significant protection in this group. We called this phenomenon increased resistance or self-defense of auditory hair cells (Graphs 1, 2 and 3).
Free radicals are produced in each cell as a by-product of normal biochemical events, especially in cellular respiration at the level of mitochondria and in abnormal processes of the body, such as inflammation, radiation, degenerative diseases, neurotoxicity, and drug adverse reactions. Gentamicin amplifies the production of free radicals of hydrogen superoxide and peroxide because it forms a gentamicin-iron complex that induces formation of free radicals, leading to damage to the outer hair cells of Corti's organ6. Free radicals could result in the production of different cell damage, as well as reduction of antioxidant or anti-free radical levels, which would be responsible for the biological process of self-defense, such as the case of glutathione, and this would lead to cell death by apoptosis. This is the destiny of outer hair cells of Corti's organ affected by aminoglycoside antibiotics. We also know that many iron chelating substances that are also anti-free radicals have demonstrated otoprotection against ototoxicity induced by gentamicin in guinea pigs, such as the case of deferoxamine20; 2,3 dihydroxibenzoate5; alpha-lipolic acid2; 2- hydroxibenzoate (salicylate)17. These studies make us believe that aminoglycoside antibiotics really chelate iron and form an ototoxic metabolite, causing the formation of free radicals that would be responsible for hair cells damage, especially to outer hair cells, resulting in ototoxicity.
The process we have just described for the first time with amikacin does not have a known mechanism. However, it suggests that the mechanism of enhanced defense of sensorial cells by the action of the first stimulus that gradually prepares the cells to improve their self defense, increasing, for example, the level of anti-free radicals; thus, by the end of 30 days, the cells would have enhanced defense and ototoprotection capacity. The cells would be prepared to defend themselves from high toxic dose of amikacin. We know that glutathione is an antioxidant molecule and that it operates, directly or indirectly, in a number of biological roles: protein synthesis, DNA, transport processes, enzymatic activity, metabolism, cell protection, decontamination. This cellular function of protection is important in tissues that generate oxygen-active or free radical substances. Glutathione was found in the inner ear21 and it protects hair cells against ototoxicity of aminoglycoside such as gentamicin, interfering in the cytototoxic mechanism10.
Based on these findings, we can suppose that the stimulating amikacin dose used in our study could, somehow, increase the defense of outer hair cells. It is known that body fluids and tissues have a system of self-defense formed by various types of substances that protect the cells against damage caused by free radicals, that is, a defense system against aggressive substances that form the normal cellular metabolism. The increase in self-defense could be a result of the increased level of glutathione that is a basic mechanism of cellular defense against ototoxicity. It is possible that increased glutathione inactivates amikacin metabolite in an enzymatic action that involves glutathione transferase or glutathione peroxidase, and the inactivation may occur also as non-enzymatic, causing a protection against the highly toxic dose of 400mg/kg/day. Other mechanisms that could participate in the mechanism of otoprotection as described by us, also considered defense elements, are enzymes glutathione peroxidase, glutathione redutase, glutathione transferase, catalase dismutase superoxide, and non-enzymatic substances, such as vitamin C and vitamin E. The co-administration of defense substances, such as anti-free radicals was made in guinea pigs that received gentamicin and there was hearing loss attenuation19, similar to the increased cellular self-defense by amikacin at 20mg/Kg/day in our study. Therefore, the present study revealed the presence of otoprotection and it could suggest the hypothesis of administration of anti-free radicals and glutathione-dependent decontamination. The administration of anti-free radicals or the increase of protective substances caused by some type of stimulation, similarly to the findings of the present study, would promote otoprotection of the cells. We should bear in mind, though, that hair cells have other protective mechanisms that could be involved in the process, such as the case of growth factors (neutrophines)9. Based on our findings, we may ask: does the process of self-defense by amikacin against damage caused by the same amikacin exist in other cells or tissues to protect against other ototoxic drugs or against non-ototoxic drugs that have unexpected side effects? We believe that if this fact happens, biochemical knowledge about the self-defense process and the substances involved would bring great benefits to medical care in preventing undesirable drug side effects, providing small doses, especially in prolonged treatments. Which factors could increase self-defense?
CONCLUSION
Since in group B (20 mg/kg/day amikacin) there were lesions compared to group A (controls) we can state that the dose of 20 mg/kg/day is not harmful to outer hair cells, administered for a 30-day period. The dose of 400 mg/kg/day amikacin applied to animals of group C during 12 weeks was highly toxic and massive cell destruction was noticed, especially in the two first turns. Less marked lesions were noticed in inner hair cells (group C). In group D, which received a dose of 20 mg/kg/day of amikacin for 30 days, and later the ototoxic dose of 400 mg/kg/day of the same aminoglycoside for 12 days, there was marked reduction of damage to outer hair cells, especially in the two first turns, which was statistically significant. Therefore, we can state that the stimulating non-harmful dose of previous amikacin protects the outer hair cells against ototoxicity of amikacin, and it was significant in both basal turns.
Acknowledgement
We would like to thank Maria Helena de Andrade for her contribution in preparing the present paper and her support in the area of statistics, and to C.A.P.E.S. CNPq and FAPESP for the financial support.
References
1. Acar, J.F.; Witchitz, J.L.; Goldstein, F.; Talbot, J.N.; Le Goffic, L. - Susceptibility of aminoglycoside-resistant gram-negative bacilli to amikacin: delineation of individual resistance patterns. J Infect Dis., (SUPPL. 134):280-5,1976.
2. Conlon, B.J; Aran, J.M.; Erre, J.P.; Smith, D.W. - Attenuation of aminoglycoside-induced cochlear damage with the metabolic antioxidant alpha-lipoic acid. Hear Res.,128(1-2):40-4, 1999.
3. Dagli, S.; Canlon, B. - The effect of repeated daily noise exposure on sound conditioned and unconditioned guinea pig. Hear Res.,104:39-46, 1997.
4. Dulon, D.; Zajic, G.; Schacht, J. - Differential motile response of isolated inner and outer hair cells to stimulation by potassium and calcium ions. Hear Res., 52:225-32,1991.
5. Ernfors, P.; Canlon, B. - Aminoglycoside excitement silences hearing. Nature Med., 2:1338-43, 1996.
6. Garetz, S.L.; Schacht, J. - Ototoxicity: of mice and men. In: van De Water, T.R.; Popper, N.A.: Fay, R.R., Eds. Handbook of auditory research vol. VII: (Clinical aspects of hearing. New York:Springer Verlag; 1996:116-54.
7. Halliwell, B.; Gutteridge, J.M.C. - Oxygen free radicals and iron in relation on biology and medicine: some problems and concepts. Arch Biochem Biophys.,246:501-14, 1986.
8. Harrison, R.V.; Shirane, M.; Fukushima, N.; Mount, R.J. - Morphological changes to the cochlea in an animal model of profound deafness. Acta Otolaryngol. (Stockh), (SUPPL.489):5-11, 1991.
9. Kimura, N.; Nishizaki, K.; Orita, Y.; Masuda, Y. - 4-methylcatechol, a potent inducer of nerve growth factor synthesis, protects turn ganglion neurons from aminoglycoside ototoxicity-preliminary report. Acta Otolaryngol Suppl:540:12-5, 1999.
10. Lautermann, J.; McLaren, J.; Schacht, J. - Glutathione protection against gentamicin ototoxicity depends on nutritional status. Hear Res.,86:15-24, 1995.
11. Lim, H.H.; Jenkins, O H.; Myers, W.; Miller, J.M.; Altschuller, R.A. - Detection of HSP 72 synthesis after acoustic overstimulation in rat cochlea. Hear Res.,69:146-50, 1993.
12. Oliveira, J.A.A. - Audiovestibular toxicity of drugs. Vol I e II p.551 Florida Boca Raton CRC Press 1989.
13. Oliveira, J.A.A.; Rossato, M.; Anselmo-Lima, W.T. - Células ciliadas isoladas em cultura: um novo modelo para estudo da ototoxicidade. Rev Bras Otorrinolaringologia, 61(1):70-8, 1995.
14. Priuska, E.; Schacht, J. - Formation of free radicals by gentamicin and iron and evidence for an iron/gentamicin complex. Biochem. Pharmacol.,50:1749-52, 1995.
15. Schacht, J. - The biochemical basis of ototoxicity. In: Rybak, L. P., ed. Otolaryngologic clinics of North America, vol. 26. Philadelphia: W.B. Saunders; 1993:845-56.
16. Sha, S.; Schacht, J. - Amplification of free radicals by gentamicin in a biological system. Assoc Res Otolaryngol.,19:30, 1996.
17. Sha, S-H., Schacht, J. - Stimulation of free radical forming by aminoglycoside antibiotics. Hear Res.,128: 112-8, 1999.
18. Sharp, P.M.; Saenz, C.A.; Martin, R.R. - Amikacin (BB-K8) treatment of multiple drug-resistant proteus infections. Antimicrob Agents Chemother.,5:435-8, 1974.
19. Song, B.B.; Schacht, J. - Variable efficacy of radical scavengers and iron chelators to attenuate gentamicin ototoxicity in guinea pig in vivo. Hear Res., 94:87-93, 1996.
20. Song, B.B.; Sha, S.; Schacht, J. - Iron chelators protect from aminoglycoside-induced cochleo- and vestibulotoxicity in guinea pig. Free Radical Biol Med.,25:189-95, 1998.
21. Usami, S.; Hjelle, OP.; Ottersen, OP. - Differential cellular distribution of glutathione-an endogenous antioxidant - in the guinea pig inner ear. Brain Res.,743:337-40, 1996.
22. Wang, H.; Priuska, E.M.; Schacht, J. - The iron embrace: exposing the secret activities of gentamicin. Abstr Assoc Res Otolaryngol,19:29, 1996.
23. Yamasoba, T.; Schacht, J.; Shoji, F.; Miller, J.M. - Attenuation of cochlear damage from noise trauma by an iron chelator a free radical scavenger and glial cell line-derived neurotrophic factor in vivo. Brain Res.,815:317-25, 1999.