Year: 2004 Vol. 70 Ed. 4 - (17º)
Relato de Caso
Pages: 551 to 554
Middle ear adenoma: case report
Author(s):
Celso Gonçalves Becker1,
Iolanda Mauro Barra2,
Hercília Helena de Oliveira Pimenta2,
Paulo Fernando Tormin Borges Crosara3,
Carlos Alberto Ribeiro4,
Lúcia Porto Fonseca de Castro4
Keywords: Key words: adenoma, middle ear, ear surgery.
Abstract:
We describe a case report of a middle ear adenoma in man with progressive conductive hearing loss, hyperemic retrotympanic mass with slow growing and undergone a radical mastoidectomy. This is a rare tumor composed of adenomatous and neuroendocrine cells. It has a large number of differential diagnoses and it is done by microscopy and immunohistochemistry. The treatment is surgical in all cases with no additional therapy only clinical follow-up, once its prognostic is favorable.
![]()
INTRODUCTION
The purpose of the present study was to report a case of middle ear adenoma in young patients with conductive hearing loss, hyperemic retrotympanic mass of slow evolution, submitted to surgical treatment by radical mastoidectomy.
LITERATURE REVIEW
Middle ear adenoma is a rare neoplasm of benign character that may present local aggressive behavior in some cases. The term middle ear adenoma was proposed by Hyams and Michaels in 1976; however, there are many different terms that reflect the controversy involving its presumable histogenesis and differentiation 1-4.
Adenoma is an epithelial tumor, comprising glandular epithelial and neuroendocrine cells that seem to originate from the middle ear mucosa or the neural crest neuroectodermis. Therefore, some authors suggested that the most appropriate term would be neuroendocrine adenoma 5-7.
Classical clinical symptoms are unilateral hearing loss, otorrhea, otalgia and tinnitus. Facial nerve affection may occur in some cases and it is an important prognostic factor.
Imaging diagnosis is based on Computed Tomography scan (CT) or Magnetic Resonance Imaging (MRI) that reveal tumor mass involving or not the middle ear ossicles. However, they do not reflect the biological nature of the tumor and present variable practical value 8.
Histological diagnosis is extremely important and it is conducted by optical microscopy, immunohistochemistry and electron microscopy. The main immunohistochemistry markers are epithelial markers (cytokeratins AE1/AE3; cytokeratin 7), neuroendocrine markers (human pancreatic anti-polypeptide, chromogranin A, anti-PP, among others), and mesenchymal cell markers (vimetin) 5, 7.
Differential diagnosis should be made with hydroadenoma, middle ear adenocarcinoma, endolymphatic sac tumors, paraganglioma, meningioma, plasmocytoma, inflammatory polyps, carcinoid tumors, middle ear aggressive papillary tumors, adenomatous neoplasm, ceruminous and glomus tumor, among others 9-12.
Treatment consists of surgery with strict postoperative follow-up and no additional therapy is recommended given that the prognosis is good and there are no reports of deaths resultant from the disease 13-15.
CASE REPORT
MCMC, 26 years old, male Caucasian patient, single, born and living in Belo Horizonte/MG, undergraduate student.
Clinical history and clinical progression
The patient was assessed by an Otorhinolaryngologist in January 2002, with clinical history of repetitive otitis on the left for two years, whose first episode was associated with peripheral facial palsy on the same side. He took antibiotics, whose specific names he did not remember, with regression of the affection. He reported occasional nasal obstruction, with periods of cough that were correlated with episodes of sinusitis. ENT examination showed hyperemia and bulging of posterior-superior quadrant of left tympanic membrane and normal right side otoscopy; septum deviation to area IV of Cottle to the left, paranasal sinuses, oropharynx, hypopharynx and larynx without abnormalities. He was treated with Amoxicillin/clavulanate and audiometry, tympanometry, fibronasolaryngoscopy and mastoid CT scan were ordered.
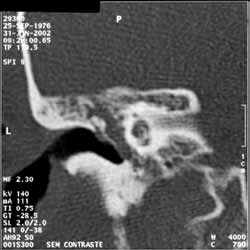
Temporal bone CT scan (Figure 1) was performed in February 2002, and it showed signals of chronic mastoiditis with middle ear velamentum. Levofloxacin was prescribed.
In March 2002, the patient had a new visit complaining of occasional sharp-pulse otalgia on the left. Otoscopy was unaltered compared to the previous test. He referred he was afraid of undergoing surgery and had not performed the ordered tests yet.
In April 2002, the patient was seen by our service and we indicated tympanomastoidectomy considering the report of otalgia for 2 years, previous history of left side peripheral palsy in the first episode, chronic mastoiditis with attical retraction, and posterior-superior bulging. We ordered new audiometry, tympanometry and surgical risk assessment and later submitted the diagnostic hypotheses and the surgical risks to the patient.
In May 2002, audiometry showed normal hearing on the right and conductive hearing loss on the left, with air-bone gap of 35 dB HL on average, in frequencies of 500, 1000 and 2000Hz. Otomicroscopy showed attic retraction, hyperemia and bulging of posterior-superior quadrant of left tympanic membrane.
Surgical management
In May 2002, the patient was submitted left radical tympanomastoidectomy under general anesthesia. We observed polyp mass occupying the whole tympanic cavity and encompassing the ossicles, and the incus was disarticulated owing to erosion of its long ramus. We collected frozen material for clinical pathology analysis. We burred the mastoid and cleaned its tip, sinodural angle and antrum which were filled with the polypoid mass and identified the lateral semicircular canal. Clinical pathology revealed very large cellular tumor of benign characteristics but aggressive, originated from the neural sheath, without aspect of palisade, suggestive of neurinoma. The pathologist recommended complete removal, if possible. We performed the surgery by burring the posterior wall of the canal and decided for radical tympanomastoidectomy. We identified a polypoid mass involving the tympanic segment and the second knee of the facial nerve, suggesting neural infiltration. We removed the mucosa and the polypoid mass from the hypotympanum, the region of the auditory tube ostium, debris of incus and malleus (which were taken by the tumor) and the whole polypoid lesion that was not directly adhered to the facial nerve. We identified remains of the supra-structure of the stapes, oval and round window. We made inversion of the margins of tube ostium, insertion of muscle fragment, sealing with bone wax, and wide meatoplasty.
Owing to absence of definite histopathological diagnosis that could provide analysis of prognosis and/or complementary treatment, and especially the need to discuss with the patient the risk-benefit correlation of an undefined pathology, we decided for not removing completely the lesions that was adhered to the facial nerve.
Clinical pathology
The material was fixed in formol at 10%, processed in histological sections through alcohol dehydration, diaphanization in xylol and blocking in paraffin. Histological sections were stained with hematoxylin-eosin and PAS.
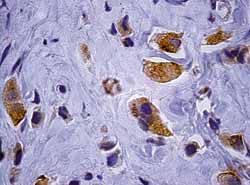
Immunohistochemistry was conducted using Labeled Streptavidin Biotin technique - Peroxidase (LSAB +), with investigation of antigens: Chromogranin A (Figure 2), epithelial membrane antigen, CD-99, GFAP (Glial Fibrillary Acidic Protein), Cytokeratin (AE1/AE3), carcino-embryonic antigen (CEA), Actin, Vimentin and Protein S-100.
Neoplastic cells were positive for Cytokeratin (AE1/ AE3), Vimentin, Epithelial membrane antigen (irregularly) and Chromogranin A, also irregularly.
Histological and immunohistochemical findings were compatible with middle ear adenoma.
Postoperative evolution
The patient was followed up for removal of sutures and weekly cleaning of the cavity in the first postoperative month and he progressed well with epithelization of the cavity and no signs of infectious process.
DISCUSSION
Middle ear adenoma is a rare tumor; preoperative diagnostic suspicion is hardly present because symptoms and audiometric findings are similar to those of most common otological pathologies, such as cholesteatomas and inflammatory polyps.
Imaging exams do not provide additional signals to suggest the etiology. They may reveal velamentum or tumor mass, affecting the ossicles or not, without necessarily causing bone erosion of Chaussé spore.
The definite diagnosis is made by histopathological and immunohistochemical exams. Middle ear epithelium shows a trend to develop glandular formation in otitis media and adenoma seems to be a benign neoplastic transformation of the epithelium.
Optical microscopy reveals regular, cuboid and columnar cells that form small, juxtaposed glands or solid arrangement of cells, or even trabecular disposition. PAS staining may be positive (mucoprotein) in the lumen of some glandular structures.
Morphological differential diagnosis is made with carcinoid tumor (low malignancy potential neoplasm) and it is impossible to make it in some occasions, given that both may present neuroendocrine peptides. Other middle ear neoplasms have different morphology, restricting differential diagnosis.
The difficulty to define better surgical approach is due to rarity of tumor and absence of standardization of its histological classification that determines tumor behavior, such as local invasion. Surgical treatment comprises tumor exeresis, with or without safety margins. Prognosis is good, and the literature recommends strict follow-up to detect recurrence.
The importance of this report is that even though rare, middle ear adenoma should be part of the differential diagnosis with masses that affect the region. We also wanted to highlight the decision that the surgeon made not to resect part of the lesion adhered to the facial nerve given that there was neither definite preoperative histological diagnosis nor prognosis information.
REFERENCES
1. Namyslowski G,Scierski W, Lange D. Middle ear adenoma: a case report. Ultrastruct Pathol 2001; 25(1):73-8.
2. Bailey QR, Weiner JM. Middle ear adenoma: a case report with ultrastructural findings. J Laryngol Otol 1986; 100(4):467-70.
3. Kundig H, Kos I, Kurt AM, Guyot JP. Adenoma of the middle ear. Ann Otolaryngol Chir Cervicofac 2000; 117(5): 274-80.
4. Hale RJ, McMahon RF, Whittaker JS. Middle ear adenoma: tumour of mixed mucinous and neuroendocrine differentiation. J Clin Pathol 1991; 44(8):652-4.
5. Davies JE, Semeraro D, Knight LC, Griffiths GJ. Middle ear neoplasms showing adenomatous and neuroendocrine components. J Laryngol Oto 1989; 103(4):404-7.
6. Ketabchi S, Massi D, Franchi A, Vannuchi P, Santucci M. Middle ear adenoma is an amphicrine tumor: why call it adenoma? Ultrastruct Pathol 2001; 25(1):73-8.
7. Gunduz M, Yamanaka N, Saito T, Kuki K, Yokoyama M, Nakamine H. Middle ear adenoma with neuroendocrine differentiation. Auris Nasus Larynx 2000; 27(1):73-6.
8. Maintz D, Stupp C, Krueger K, Wustrow J, Lackner K. MRI and CT of adenomatous tumours of the middle ear. Neuroradiology 2001; 43(1): 58-61.
9. Zeise K, Kaschke O, Jautzke G. Middle ear adenoma. Long-term course of a rare neoplasm. J Laryngol Otol 2001; 115(3):216-9.
10. Ribe A, Fernandez PL, Ostertarg H, Claros P, Bombi JA, Palacin A et al. Middle-ear adenoma (MEA): a report of two cases, one with predominant "plasmacytoid" features. Ann Pathol 1996; 16(4):271-5.
11. Mc Donald KR, Vrabee JT. Synchronous middle ear osteoma and adenoma. Surg Neurol 1997; 48(4):368-73.
12. Caldas Neto S, Depart A, Bento RF, Caldas N. Tumours of the ceruminous glands: revision of the literature and report of a case in the middle ear. Rev Laryngol Oto Rhino (Bord) 1993; 114(1):43-7.
13. Wassef M, Kanavaros P, Nemeth J, Adjamagbo H, Ba Huy PT. Amphicrine adenoma of the middle ear. Histological, immunohistochemical and ultrastructural study of a case. Ann Pathol 1993; 13(3):170-5.
14. Torske KR, Thompson LD. Adenoma versus Carcinoid Tumor of the Middle Ear: a Study of 48 Cases and Review of the Literature. Mod Pathol 2002; 15(5):543-55.
15. Paraskevakou H, Lazaris AC, Kandiloros DC, Papadimitriou K, Adamapoulos G, Davaris PS. Middle ear adenomatous tumor with a predominant neuroendocrine component. Pathology 1999; 31(3):284-7.
Figure 1. Temporal bone CT scan, left side, coronal section, demonstrating velamentum of middle ear.
Figure 2. Adenoma immunohistochemistry: presence of neoplastic cells identified by Chromogranin A (1000x).