Year: 2004 Vol. 70 Ed. 4 - (7º)
Artigo Original
Pages: 484 to 493
The role of middle fossa approach in management of traumatic facial paralysis
Author(s):
Ricardo Ferreira Bento1,
Sulene Pirana2,
Rubens Vuonno de Brito Neto3,
Tanit Ganz Sanchez4,
Arthur Menino Castilho5,
Robert Carl Sweet6
Keywords: Key words: facial palsy, trauma, middle fossa.
Abstract:
Introduction: Management of traumatic facial paralysis has several controversial aspects. One of these is the precise nature of surgical intervention to be used once the decision to operate has been made. Aims and Methods: Between June 1984 and June 1993, 220 cases of traumatic facial paralysis with good cochlear reserve were treated at the University of São Paulo by the following surgical approach: decompression of the tympanic and mastoid segments by the transmastoid approach and decompression of the geniculate ganglion and the distal 50% of the labyrinthine segment by the middle fossa approach. We present a review of the literature and a discussion and results of our management.
![]()
INTRODUCTION
Middle fossa approach (MFA) through anterior-posterior surface of petrous pyramid has been used for the removal of small acoustic neuromas, for selective vestibular neurectomy and to decompress and repair geniculate ganglion and labyrinthine portion of facial nerve. Other less common indications include petrous apex damage, facial nerve schwannoma, cerebrospinal fluid (CSF) leaks, and temporal lobe encephaloceles, and more currently, cochlear implants in ossified cochlea. Its use has been extended to the division of upper petrous sinus to remove large-sized acoustic neurinomas and petrous-clival meningeomas. There is also the intradural middle fossa approach used by neurosurgeons. Extradural, non-extended approach through inferior temporal craniotomy, as described by House1, is the object of the present paper. The approach is unique because it allows direct exposure of the bottom of the inner auditory canal and labyrinthine portion of the fallopian canal with preservation of inner ear function. It is also unique in exposing the pre-ganglionar region of facial nerve. MFA, however, has been associated with serious complications. To some, this approach has been used as an indispensable tool whereas to others, there seems to be no rationale for its use.
The success of posterior approaches to vestibular neurectomy and neurinoma surgery with preservation of hearing has reduced the frequency of MFA indications to this end. There is still controversy concerning efficiency of the procedure for decompression of facial nerve in Bell's palsy, which has discouraged many otologists from performing the surgery. However, there are fewer questions concerning the use of middle fossa approach in cases of traumatic facial palsy (TFP) 2.
It is widely acceptable and reasonable that total TFP, with loss of lachrymation and significant reduction or absence of electrical response but preserved hearing, be treated with MFA 3. The present paper concentrated on patients that have this amplitude of signs and symptoms, the typical patient that suffered temporal bone fracture. Even in this topic, there is some controversy and disagreement, especially concerning possible complications of MFA rather than its efficiency.
Salaverry4 described in 1974 the approach of geniculate ganglion and the first segment of facial nerve through transattical approach, which represents the only possible alternative to access in this region through mastoid opening.
The subjects of the present study were those submitted to this approach for decompression, anastomosis or grafting of facial nerve trauma. Conversely, complications that concern physicians and patients, especially neurological effects, which have never been addressed by the literature, are going to be discussed in details.
In the Department of Otorhinolaryngology, University of Sao Paulo, MFA is used mainly for management of TFP after closed cranial trauma, and for the past 10 years significant experience has been accumulated. The purposes of the present study are:
1. to review our experience;
2. to assess complications and the role of MFA in management of TFP.
MATERIAL AND METHOD
Patients
Medical charts of patients with TFP operated on by the main investigator over the past 10 years were reviewed and inputted in a computer database. Those operated on using MFA were analyzed concerning preoperative, surgical and postoperative period variables.
LITERATURE REVIEW
A review of MEDLINE, LILACS, Revista Brasileira de Otorrinolaringologia, Arquivos da Fundação Otorrinolaringologia and Acta AWHO related to key words traumatic facial paralysis and middle fossa for the past 10 years was conducted, followed by selection of the most relevant articles.
Terminology
At the bottom of the inner ear canal, facial nerve goes into fallopian canal through the meatal foramen. The labyrinthine portion of fallopian canal extends to meatal foramen up to geniculate ganglion; distally, they direct axons to innervating facial expression muscles, in the labyrinthine, pyramid and mastoid portions. The fallopian canal ends in the stylomastoid foramen, in which the nerve emerges from the skull and starts its extracranial portion 5.
The pre-ganglion portion (PG) includes the geniculate ganglion and its surrounding region. It is not precisely defined; however, it does not include the meatal foramen, proximal half of labyrinthine portion of facial nerve or distal half of tympanic portion. Fisch6-8 is believed to have created this terminology of meatal foramen, which demonstrates the narrowest portion of fallopian canal. He also described the phenomenon of nerve compression in critical regions, which can be significant in Bell's facial palsy or TFP.
Management of TFP at University of Sao Paulo
Protocol
Facial nerve traumatic damage is explored as emergency and patients are submitted to end-end anastomosis of facial nerve or complete nerve grafting, be it contaminated or cleaned injury 9.
Paralysis associated with mandible fracture is treated by wait and see management, as well as palsy associated with birth trauma; exploration is reserved to cases of poor evolution, without restoration of function after 6 months.
If possible, iatrogenic lesions are repaired in the same surgical act or as quickly as possible after the surgery that caused the facial palsy. In general, none of the above referred situations or others in which there is labyrinthine portion damage require MFA.
Patients that had significant head trauma with associated FP, be it by closer trauma or firearm injury, are submitted to reanimation measures, medical, surgical and neurosurgical assessment before being submitted to facial nerve assessment. Clinical ENT history and examination should be conducted, with special emphasis on facial and other cranial nerves. A standard form is filled out and data are inputted into an electronic database. Audiometry, electroneuromyography, laboratory exams and high resolution computed tomography are conducted.
Firearm injury can be related to suicidal attempts. Thus, a consultation with psychiatrist should be made. Surgical wound infections are common in these cases and should be aggressively treated. If the surgery is indicated based on closed trauma criteria, MFA is rarely used. If it is an infra-geniculate lesion, mastoid access is enough, whereas if it is a supra-geniculate lesion, hearing is invariable destroyed and translabyrinthine approach is preferred.
In closed trauma, concussion and temporal bone fracture, if palsy is total and electroneuromyography shows severe degeneration, the decision to conduct surgery is made as soon as the patient is released by the neurosurgery team, even if the history shows late palsy or computed tomography (CT) does not show fracture or non-leveling of Fallopian canal. The approach is directed by topographic information (lachrymation test) or audiometric information: if infra-ganglion, we use mastoid approach; in supra-ganglion damage with anacusis (ex. transversal fracture), translabyrinthine approach, and in supra-ganglion damage with good cochlear reserve (ex. longitudinal fracture), we prefer a combination of MFA and transmastoid approach.
Surgical technique for patients submitted to MFA
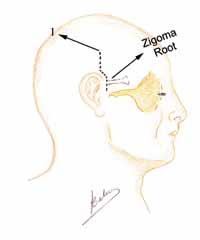
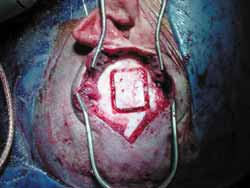
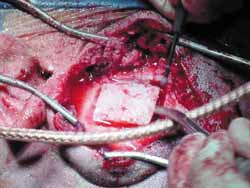
The surgery is conducted under general anesthesia, the patient is placed on supine position and the head is turned. Mastoidectomy is conducted before and consists of standard decompression of facial nerve, stylomastoid foramen up to geniculate ganglion, with opening of sheath and reconstruction of ossicle chain. The middle fossa is approached through extension of retroauricular incision (vertical expansion: Figures 1 and 1a), and then a bone window of 3cm (anterior-posterior) by 4cm (upper-lower) is made, centered on the root of zygomatic arch (Figures 2 and 3).
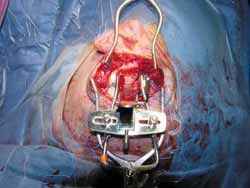
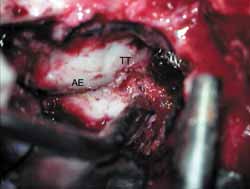
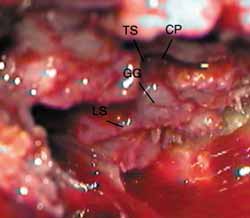
The inferior margin of craniotomy is lowered using a drill. Intracranial pressure is reduced with hyperventilation or use of mannitol (diuresis) or CSF drainage through a small dura opening. Dura is elevated and the lateral-superior surface of petrous portion of temporal bone is exposed (Figure 4). Dura can be involved in the fracture line. A modified Urban-Brackmann expander is placed to expose facial hiatus, arcuate eminence and meatal plan (Figure 5). In cases of open mastoid, translumination through mastoid cavity can help orientation. Geniculate ganglion (Figure 6) is located by identification of tympanic tegmen and cochlearform process, facial hiatus and followed posterior-laterally with diamond bur and constant suction/irrigation 10. The labyrinthine portion of Fallopian canal is followed progressively, but not in its whole extension, up to the inner auditory canal (Figure 7). Thus, pre-ganglionar region is smoothly explored. The 1-2cm proximal to the labyrinthine portion and inner acoustic canal are not explored, unless there is damage in these areas (Figures 8 and 9). Next step, tympanic portion is explored up to finding the exposure obtained via transmastoid approach. Final fragments of the bone are removed from the nerve and the incised sheath. Pre-routing or graft repair is rarely necessary in longitudinal fractures. Petrous nerve is sectioned and cauterized with bipolar device to prevent regeneration of facial nerve axons through this approach. It does not normally cause harmful effects to ocular lubrication.
Middle fossa and attic communication is not repaired. Any significant lesion to dura is repaired primarily or closed with fascia or biological glue. After conducting hemostasis, the retractor is removed, allowing the brain to re-expand. Dura is then repaired with craniotomy using non-absorbable thread and bone fragments previously removed, and then sutured in anatomical position. The surgical wound is closed in planes and a suction drain is placed in the subgaleal space. Prophylactic antibiotic therapy with 4th generation cephalosporin is used as routine.
RESULTS
In the past 10 years, from June 1984 to July 1993, 220 patients were treated of traumatic facial palsy. This series of patients included findings of 156 patients with over 90% degeneration of facial nerve (electroneurography), good hearing and supra-geniculate lesion.
Systemic corticoid therapy was the preoperative management used with these patients.
The age of patients ranged from 4 to 70 years (mean age 33 years), and there were 43% female and 57% male patients. In 44 cases (31%), the cause was accidental fall, in 29 (21%) motorcycle accident, in 48 (34%) car accident, in 16 (11%) assault, in 2 (1%) knife wound, in 1 (11%) firearm wound, and in 2 (1%), undetermined cause.
Facial palsy started immediately in 150 cases (96%). The right side was affected in 81 (52%) cases and the left side in 73 (47%), being bilateral in 2 (1%).
In 74 patients (45%), otoscopy was normal, in 78 (46%) there were signals of bleeding, in 11 (7%) there were infections, in 1 (1%), external auditory canal stenosis, and in 1 (1%), there was tympanic membrane perforation.
In 36 cases (23%), audiometry was normal, in 110 (71%) there was air-bone gap, and in 10 (6%), there was mixed hearing loss.
In 114 (73%) patients we could detect fracture by CT scan. It was longitudinal in 98 cases (62%), transversal in 12 (8%), and multiple in 4 cases (3%).
The preoperative function of facial nerve was clinically assessed using House-Brackmann scale 11. Out of 156 cases, 3 (2%) had moderate to severe palsy (IV), 138 (88%) had severe palsy (V) and 15 (10%) had total palsy (VI). In one case only, there was damage to other cranial nerves rather than 7th and 8th nerves, affecting the 6th cranial nerve.
MFA was conducted in a period that ranged from 6 to 240 days (mean of 45 days) after trauma. The fracture was observed intraoperatively in 138 cases (88%). It was on the middle fossa floor (over the geniculate ganglion) in 63 cases (40.03%) and on the first portion of facial nerve in 75 (48.07%). In 147 (94%), there was facial nerve edema, in 4 (3%), partial section, in 1 (1%) hematoma, and in 8 (5%) there was no facial nerve laceration.
All patients were treated with antibiotics and corticoids. Postoperative complications are listed in Table 1. Twenty patients (13%) had vertigo, which was solved some days later using labyrinth sedatives.
Postoperative assessment was also made, using House scale after 1 year. Sixty-eight subjects (43%) had normal function of facial nerve (I), 57 (37%) had mild dysfunction (II), 19 (12%) had moderate dysfunction (III), 11 (7%) had moderately severe (IV) and 1 (1%) had severe dysfunction (V).
DISCUSSION AND CONCLUSIONS
Since the 70's, the apparent popularity of MFA has been decreasing 12-14 owing to lack of understanding of the pathophysiology of diseases that would require the use of this approach, development of other approaches, and complications resultant from it. Even if this reduction is justified by approaches to acoustic neurinoma and Ménière's disease (neurectomy), it should not be applicable to cases of TFP. In fact, our experience, as well as others' 15-18, suggest that this approach can be safe and effective to traumatic facial palsy.
Regardless of treatment, risk-benefit correlation and alternative management approaches can be determined and discussed with patients before the definition of final treatment. This issue takes on special importance for elective and semi-elective procedures in intracranial surgery, such as MFA in TFP. Informed consent can be a problem to this group of patients, given that some of them could have resulted from a situation of coma and others present neurological damage which prevent them from making such a decision. Some can even have post-concussive syndrome. We assume the benefits of this surgery in such cases in order to reach House scale levels I and II10, but this is a topic that is widely debated and it is not the scope of our present study. In order to illustrate some of these aspects, we can say that:
a) There is no consensus in the literature addressing efficacy of surgery in closed nerve trauma in general;
b) The influence of the peculiar anatomy of facial nerve and its response to trauma and surgery are still under study;
c) The results depend on nature, extension and time of surgery;
d) The possibility of intracranial facial nerve lesion, particularly at its emergence from the root, has been obscured by the intratemporal trauma per se;
e) There is lack of prospective, double-blind and controlled studies on the topic.
Let us analyze the complications of MFA, surgical alternatives to pre-ganglionar region and, finally, some non-medical factors that can affect MFA in otological practice.
Complications of MFA
They can be divided into those that can theoretically occur and those that were actually reported in surgical practice (Table 1).
1. Anesthetic:
In addition to usual general anesthesia risks, there may be associated complications with induction of hypocapnia, osmotic diuresis and controlled hypotension and hypovolemia (such as cerebral ischemia, cerebral edema, hemolysis and renal failure).
2. Patient's position:
If the patient is on supine position, the head and neck should be rotated. Depending on intensity of cervical movement, there may be torticollos or quadriplegia. Arterial and venous vertebral flow can be equally impaired, leading to consequences such as cerebral ischemia and intracranial pressure increase. If the head is elevated to promote venous drainage and reduce intracranial pressure, there may be upper petrous sinus embolia.
3. Incision and craniotomy:
Early complications include subgaleal collection of blood or epidural hematoma. Surgical wound infection may occur, leading to abscess. Late complications include: esthetical problems, with loss of hairs on the incision line, visible scar and atrophy of temporal muscle, headache, localized pain and edema, temporomandibular joint problems, and reabsorption of bone graft. If the incision is anterior-inferior, there may be laceration and compression of facial nerve temporal ramus.
4. Dura elevation:
Dura laceration is more common in elderly subjects, in which dura tends to be thinner and more adhered to the underlying bone, but it may also occur at any age in patients who have temporal bone fracture, owing to the tendency the dura has to being attached to fracture lines. These lacerations can cause CSF leaks and meningitis, especially when mastoid cells are opened.
Dura elevation may cause bleeding from temporal bone roof small cells. Similarly, there may be bleeding from middle meningeal artery. As a result, epidural hematoma may occur in the postoperative period. It may, in turn, lead to emergency life-threatening conditions and the need for immediate opening of surgical wound and drainage of collection. Epidural subacute collections may occur, with potential for formation of epidural abscess. Dura elevation may damage geniculate ganglion and petrous nerves and arteries, given that in some situations these structures may be adhered to the dura.
5. Temporal lobe retraction:
During surgery, temporal lobe retraction can lead to increase in intracranial pressure and push it, causing compression of brainstem. Postoperatively, the damage caused by retraction can lead to cerebral edema, increase in intracranial pressure, brainstem compression and later, encephalomalacia with deficits to temporal lobe and epilepsy. Retraction can also lead to laceration of ligant veins and bleeding in subdural and subarachnoid spaces.
Pathophysiology of damage by ischemic retraction
Based on Ohm's law, cerebral blood flow (FSC) is proportional to cerebral perfusion pressure (PPC) divided by cerebrovascular resistance (RC), ex: FSC = PPC/RC. RC is normally controlled by cerebrovascular auto-regulation to maintain FSC constant, regardless of PPC changes. However, auto-regulation, which is beyond physicians' control, can be deficient, especially after trauma. Thus, it is important to maintain appropriate and stable PPC. Global PPC is approximately average arterial pressure (PAM) minus intracranial pressure (PIC), ex: PPC=PAM-PIC. Similarly, PPCr= PAM-PRC, in which PPCr is regional perfusion pressure and PRC is cerebral retraction pressure. What does it mean? In practical terms, whereas the anesthesiologist controls PAM, the surgeon has to be attentive to very intense pressure of the retraction lobe, dispensing strict control of PIC. Objective monitoring to avoid damage by retraction is feasible, but it is not used as routine. A reduction of PIC can be safely used with hypocapnia induced by hyperventilation, by pCO2 of 25mmHg (to reduce cerebral volume), supplemented if necessary by use of diuretics, or removal of CSF using lumbar puncture or small dura opening.
Patients with head trauma present a special problem owing to the already existing cerebral edema, intracranial hematoma or auto-deregulation which can lead to impaired cerebral relaxation. The cerebral edema peak takes place at about the first week after the trauma 19. Wait for the patient to improve from acute problems does not ensure safety, since subdural hematoma and hydrocephalus can develop later; head CT scan can exclude these conditions 20, 21.
Ischemic lesion is not related only to increase in pressure, but also to duration of retraction. Animal studies showed that the threshold for healthy cerebral tissue infarction is PCR of 20mmHg for 1 hour. Minor procedures, such as decompression of geniculate ganglion, tend to cause less retraction lesion than longer procedures.
Clinical characteristics of temporal lobe lesion
Retraction lesion can produce temporary cerebral edema or permanent infarction, with visible encephalomalacia in head CT scan or magnetic resonance imaging. Some functional deficits or convulsions may occur, even though in intact dura the risk of epilepsy is reduced. The temporal lobe is important for hearing, learning, memory, emotions and integration of multi-sensorial functions with past experiences. The dominant side (normally the left one) is important for language (verbal language and memory), whereas the non-dominant side is more related with spatial notions and artistic concepts (non-verbal learning and memory). MFA retractor probably compresses the inferior temporal gyrus and the spindle gyrus, corresponding to Brodman areas 20 and 21, which are non-specifically associated with the cortex. This region is relatively far from the primary hearing cortex and limbic structures, but on the dominant side, it may come close to the posterior margin of the speech area of Wernicke. Minor deficits can be discreet and may take to moderate difficulties such as recent memory, naming objects or reading impairment. More significant deficits can lead to aphasia, cognitive, behavioral and psychiatric problems.
Temporal lobe epilepsy, previously known as psychomotor epilepsy, is a condition in which there are recurrent seizures. The aura can be characteristic: auditory, olfactory, or gustative hallucinations, deja-vu or jamais-vu, and anxiety and fear. The patient may get into a state of abnormal consciousness with abnormal repetitive behaviors, called automatic behaviors, which the patient cannot remember. In some cases there are no convulsions and the state can be mistaken by psychiatric disorder.
6. Main part of surgery:
The main part of the surgery is focused on the risk condition, which will depend on type, location and extension of pathology. The risk of MFA for the removal of acoustic neurinoma, vestibular nerve section and facial nerve decompression differs since the likelihood of anterior-inferior cerebellar artery damage, in the inner acoustic canal (the loop it makes), is high in neurinoma surgery and vestibular nerve section, but not in pre-ganglionar region exploration. This lesion can lead to subarachnoid hemorrhage and brainstem or cerebellum infarction. Direct damage to the brainstem and other cranial nerves (others than 7th and 8th) should only occur in surgeries of cerebellum-pontine angle or petrous-clival lesions.
7. Closing:
If there is significant non-repaired defect in tegmen, there may be encephaloceles (herniation of cerebral tissue). It may cause conductive hearing loss or pulsatile tinnitus by contact with ossicle chain 22, 23. As time goes by, gravity defects may thin the dura of the region with encephaloceles and cause CSF leaks, meningitis and convulsions 24, 25.
To suture dura, be it to correct a laceration or to fix it (to close epidural space), may cause bleeding in the subdural or subarachnoid space.
Comments and recommendations about complications
Out of all theoretical considerations shown and reported in studies, the risk of death or neurological damage can be greater in cases of acoustic neurinomas, reducing progressively in procedures such as vestibular neurectomy, TFP and Bell's palsy 26.
The risk of subdural hematoma is unpredictable, requiring studies of preoperative coagulation and hypertension control and close postoperative follow-up of any middle fossa procedure.
The risk of lesion by retraction is greater in patients with head trauma, elderly patients with cerebrovascular diseases and prolonged procedures. To reduce the risk of patients with head trauma, it is better to wait for the cerebral edema to be solved, PIC and auto-regulation system to be normalized and chronic subdural hematoma and hydrocephalus to be treated. During surgery, we should be as brief as possible in terms of retraction, maintaining its intensity. In addition to cases of convulsion reported by Glasscock 15, 27, we did not find data on temporal lobe epilepsy or deficit. Many authors emphasized lack of severe or permanent neurological deficits. However, temporal lobe dysfunction may be left unnoticed. There are no studies reviewed in the literature that show the possibility of encephalomalacia or sudden neurological deficits. They can, in turn, be identified with the use of CT scan, MRI and neuropsychological or central hearing tests. Many patients with acoustic neurinoma have postoperative imaging tests, and long-term postoperative follow-up is expected in patients submitted to vestibular neurectomy. Conversely, patients that are victims of trauma can have temporal lobe damage owing to the accident itself, hindering the detection of lesions.
Despite the theoretical risks of medullar and cervical damage, none have been reported. This is also our experience and that of other otologists after many years of otological surgery.
Exploration restricted to PG region leads to reduced risk of damage to hearing, balance and anterior-inferior cerebellar artery compared to proximal exploration of labyrinthine portion or inner auditory canal.
CSF leaks are more prevalent with opening of large aired cells, and even though not all authors agree on the correlation between CSF leaks and meningitis 5, in our cases all CSF leaks where followed by meningitis. Temporal lobe herniation does not seem to cause great difficulties in practice.
Alternative access to proximal intratemporal facial nerve
As reported before, MFA allows better exposure of pre-ganglionar region. Otologists suggest there is exposure of the same area through other approaches, especially in cases of benign pathologies.
Salaverry4, inspired by Lempert's surgery to access the petrous apex, developed transattical approach in 1974 primarily designed to make complete decompression of facial nerve in Bell's palsy, leaving MFA to TFP that require grafts. May28 described transmastoid, extralabyrinthine and subtemporal approaches in 1979, which differentiated from the approach described by Salaverry in the use of retroauricular incision rather than endoaural incision and disarticulation, rotation and repositioning of incus rather than removal of malleus head. At that time, decompression of the most proximal intratemporal portion of the facial nerve was widely used for Bell's facial palsy.
It was logical to expand the indication of this approach to treat TFP in longitudinal fractures and the use of translabyrinthine approach in transversal fractures, eliminating the need to use MFA. However, traumas are different from Bell's palsy, and in our opinion, they require wider exposure. It may not be enough to approach the nerve and check its integrity. In some cases, repairing it is also required.
In a study by Goin29, who compared exposure in May's procedure and MFA, they found that the former can expose the distal portion of labyrinthine portion, but it does not expose more proximal areas (only possible in 1/3 of the times). Thus, if the surgery requires exploration and decompression of meatal foramen and proximal labyrinthine portion, MFA should be used. If there is need to repair pre-ganglionar region, there will be more space for manipulation and better angle with MFA. However, if only exposure and decompression of pre-ganglionar area are required, the transattical approach can be used.
Fisch7 regularly uses MFA in facial palsy associated with longitudinal fractures because he believes in the importance of meatal foramen decompression not only in Bell's facial palsy but also in trauma. Lambert and Brackmann15, in a study of 26 cases of the same category, used May approach 5 times but required MFA for the remaining cases. It is not known whether they decompressed the metal foramen or not. Glasscock30-32 identified the geniculate ganglion and continued labyrinthine follow-up up to the inner acoustic canal using MFA; Cocker33 noticed that depending on extension of cerebral damage, MFA can be preferred for May's approach.
In our opinion, once the phenomenon of meatal foramen compression exists, the result of associating decompression of the area with exploration and repair of pre-ganglionar (and distally) region in longitudinal fractures does not justify the additional risk to inner ear structures.
At the present moment, our philosophy with these patients is to restrict exploration of PG regions to the nerve, where fractures can normally be found, and not to decompress the meatal foramen. We prefer MFA because we believe that it is justifiable to have superior exposure in this critical area, provided that there are no neurosurgical contraindications.
Non-medical factors
There are some real and unreal factors that have negative impacts on MFA in otological practice. Some have already been mentioned and others are quite obvious. For example, the need to have a neurosurgical team on call. Some authors also think it is not comfortable to work over the head position of the patient. Others tend to believe that it is hypocritical to advocate a surgery in which there is cerebral retraction, considering there are other options.
It does not matter how effective the theory is in practice, or even what is the experience of others, because if the surgeon does not feel comfortable to perform the procedure and does not have the necessary infrastructure, it does not matter whether the procedure is easier or more difficult than others.
These factors and specially those concerning the vision the patients have about the procedure are real and should be accounted for in the final equation that is going to decide what procedure is going to be adopted. Paraphrasing American singer Paul Simon, the tegmen to a surgeon is the roof to another.
In our experience, MFA showed satisfactory results in selected cases of TFP. The exposure of PG region provides satisfactory results and minimum complications.
The considerations of the literature and the theory suggest that the expected neurological complications are minor in vestibular neurectomy and especially in the exeresis of acoustic neurinoma using the same approach. However, special attention should be given to prevent increase in intracranial pressure and damage by retraction (more susceptible in patients with head trauma, especially in early post-trauma conditions).
The risk of otological complications can be reduced if exploration is confined to PG region and is not taken to proximal labyrinthine portion and inner acoustic canal.
We believe that MFA has a definite role in management of TFP, since it allows good exposure of the most commonly involved areas. We also think that the risk-benefit correlation is favorable to the patient, even though undergoing studies will determine the benefits of facial surgery in continuous lesion of this nerve. Despite the fact that cost-benefit ratio is favorable in most cases, factors that are not directly related can reduce the role of other factors.
We have used MFA for facial palsy cases after closed trauma in which there was poor prognosis defined by electroneuromyography, evidencing geniculate or supra-geniculate involvement and good cochlear reserve. In the future, indications can be expanded to include cases that have residual auditory function or anacusis with intact vestibular function. Moreover, if decompression of meatal foramen is necessary, we should think about the possibility of MFA considering the good exposure this approach provides. Conversely, this approach can be less important if: a) the use of MRI improves precision of images of the damage, especially in the emergence of the nerve in its root; b) experimental and controlled studies show that facial nerve surgery does not improve the results of continuous lesions; or, c) cases of encephalomacia, sudden neuropsychological deficit, or temporal lobe epilepsy owing to temporal lobe retraction become evident. However, to present, we consider these possibilities as very unlikely.
REFERENCES
1. House WF. Surgical exposure of the internal auditory canal and its contents through the middle cranial fossa. Laryngoscope 1961;71:1363-85.
2. Bogar P, Bento RF. Paralisia facial de origem traumática. Arquivos da Fundação Otorrinolaringologia 1997; 1: 23-5.
3. Bento RF, Miniti A, Ruocco JR. Traumatic peripheral facial palsy: diagnosis, etiology and treatment. In: 5th Facial Nerve Symposium - Bordeaux 1984. Proceedings. Paris, Masson, 1985; p299-303.
4. Salaverry MA. Transattical approach. A technique variation for total decompression of the facial nerve. Rev Bras Otorhinolaryngol 1974; 40:262-4.
5. Catalano PJ, Eden AR. An external reference to identify the internal auditory canal in middle fossa surgery. Otolaryngol Head and Neck Surg 1993; 108:111-6.
6. Fisch U. Neurectomy of the vestibular nerve. Surgical technique: indications and results obtained in 70 cases. Rev Laryngol Otol Rhinol 1969; 90:661-72.
7. Fisch U. Facial Palsy. [audiotape] 42nd Annual Midwinter Clinical Convention of the Los Angeles Research Study Club. Auto-Digest Otorhinolaryngol April 1973; 6:8 side B.
8. Fisch U. Vestibular neurectomy, in: Silverstein H, Norrell H (eds.): Neurological Surgery of the Ear. Birmingham, Aesculapius Publishing Co (1977).
9. Bento RF, Voegels RL, Brito Neto RV, Sanchez TG. Traumatic Intratemporal Facial Nerve Paralysis: Analysis of 23 cases. Otology and Neurotology 2002; 23(3) Suplement:S59.
10. Bento RF, Sanches TG, Brito Neto, RV. A rapid and safe middle fossa approach to geniculate ganglion and labyrinthine segment of the facial nerve. ENT Journal 2002; 81:320-26.
11. House JW. Facial nerve grading system. Laryngoscope 1983; 93:1056-69.
12. Shelton C, Brackmann DE, House WF, Hitselberger WE. Middle fossa acoustic tumor surgery: results in 106 cases. Laryngoscope 1989; 99:405-8.
13. Shelton C, Hitselberger WE. The treatment of small acoustic tumors: now or later? Laryngoscope 1991; 101:925-8.
14. Gavilán J, Gavilán C. Middle fossa vestibular neurectomy. Long-term results. Arch Otolaryngol 1984; 110:785-7.
15. Lambert PR, Brackmann DE. Facial paralysis in longitudinal temporal bone fractures: a review of 26 cases. Laryngoscope 1984; 94:1022-6.
16. Zini C, Mazzoni A, Gandolfi A, Sanna M, Pasanisi E. Retrolabyrinthine versus middle fossa vestibular neurectomy. Am J Otol 1988; 9:448-50.
17. Goertzen W, Christ P. [Diagnosis and treatment of lesions of the facial nerve after fracture of the temporal bone.] Rev Laryngol Otol Rhinol (Bord) 1990; 111:33-6. French.
18. Fisch U. Management of intratemporal facial nerve injuries. J Laryngol Otol 1980; 94:129-34.
19. Fernandes CM, Earle JW. Deep temporal bone surgery for traumatic facial palsy. S Afr J Surg 1983; 21:77-82.
20. Harker LA, McCabe BF. Iowa results of acoustic neuroma operations. Laryngoscope 1978; 88:1904-11.
21. Haid CT, Wigand ME. Advantages of the enlarged middle cranial fossa approach in acoustic neurinoma surgery: A review. Acta Otolaryngol (Stockh) 1992; 112:387-407.
22. Garcia-Ibañez E, Garcia-Ibañes JL. Cirugía del vértigo - neurectomía vestibular por fosa medía, in: Garcia-Ibañes E, Garcia-Ibañes JL (eds.): Cirugía del conducto auditivo interno. XVIII reunion anual de la Sociedad Espanõla de Otolaringología. Acta Otorrinolaringol Español. 1973; año24, vol.5. London, Madrid: F. García Sicilia 1973:207-11.
23. Garcia-Ibañes E, Garcia-Ibañes JL. Middle fossa vestibular neurectomy: a report of 373 cases. Otolaryngol Head Neck Surg 1980; 88:486-90.
24. Lalwani AK, Jackler RK, Harsh GR 4th, Butt FY. Bilateral temporal lobe encephaloceles after cranial irradiation. Case report. J Neurosurg 1993; 79:595-9.
25. Hyson M, Andermann F, Olivier A, Melanson D. Occult encephaloceles and temporal lobe epilepsy: developmental and acquired lesions in the middle fossa. Neurology 1984; 34:363-6.
26. Jenkins HA, Herzog JA, Coker NJ. Bell's palsy in children. Cases of progressive facial nerve degeneration. Ann Otol Rhinol Laryngol 1985; 94(4 Pt 1):331-6.
27. Glasscock ME 3rd, Miller GW. Middle fossa vestibular nerve section in the management of Menière's disease. Laryngoscope 1977; 87:529-41.
28. May M. Total facial nerve exploration: transmastoid, extralabyrinthine and subtemporal. Indications and results. Laryngoscope 1979; 89(6 Pt 1):906-17.
29. Goin DW. Proximal intratemporal facial nerve in Bell's palsy surgery. A study correlating anatomical and surgical findings. Laryngoscope 1982; 92:263-72.
30. Glasscock ME. Chronic petrositis: diagnosis and treatment. Ann Otol Rhinol Laryngol 1972; 81:677-85.
31. Glasscock ME, McKennan KX, Levine SC. Acoustic neuroma surgery: the results of hearing conservation surgery. Laryngoscope 1987; 97:785-9.
32. Glasscock ME 3rd, Wiet RJ, Jackson CG, Dickens JR. Rehabilitation of the face following traumatic injury to the facial nerve. Laryngoscope 1979; 89:1389-404.
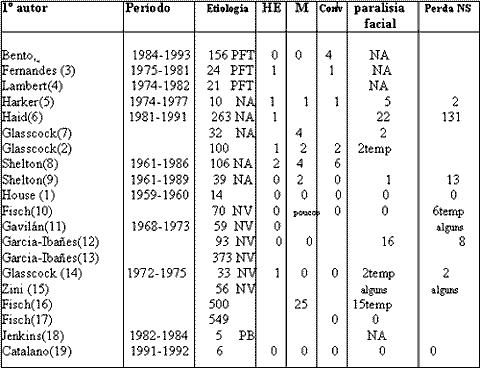
33. Coker NJ. Management of traumatic injuries to the facial nerve, in Weisman RA, Stanley RD (eds.): Current Issues in Head and Neck Trauma. Otolaryngol Clin North Am 1991; 24:215-27.Table 1. Report of complications using middle fossa approach.
Blank space means there were no reports of complications. O indicates that it was reported and there were no complications; HE = epidural hematoma; M = meningitis; Conv = convulsion; NSH = sensorineural hearing loss; NA = acoustic neurinoma; tem = temporary; NV = vestibular neurectomy; PFT = traumatic facial palsy (normally caused by temporal bone longitudinal fracture); PB = Bell's facial palsy; NA = not applicable.
Figura 1.
Figura 1a.
Figura 2.
Figura 3.
Figura 4.
Figura 5.
Figura 6.
Figura 7.
Figura 8.
Figura 9.