Year: 2002 Vol. 68 Ed. 6 - (12º)
Artigos Originais
Pages: 851 to 858
Otoacoustic emissions and ABR: study in hyperbilimbinemics newborns
Author(s):
Francisco Sales de Almeida 1,
Paulo Roberto Pialarissi 2,
Afonso Celso Monte Alegre 3,
José Vitor da Silva 4
Keywords: jaundice/ brainstem injury, jaundice/ deafness, jaundice/ otoacoustic emissions, jaundice/ brainstem audiometry
Abstract:
Bilirubin, when in high serum concentrations, causing jaundice, may impair the peripheric and central nervous system and also adhere to it. Aim: The objective of this study is to evaluate the hearing system of the icteric newborn, at term, adequate for his/her gestational age, also specially to identify `the alterations in the cochlea functions, brainstem and to compare the disorders found in the hearing system with serum concentration of bilirubin. Electrophysiologic procedures to evaluate the hearing system by receiving otoacoustic emissions and brainstem auditory evoked responses were adopted. Study design: Clinical prospective. Material and method: Thirty-two icteric newborns, presenting serum concentrations of bilirubin higher than 10 milligrams per deciliter or at the peak of its concentration, were evaluated and reevaluated at the time of its normalization. Result: The variables representing the receiving of brainstem auditory evoked responses, amplitude of waves I and V, interpeak latencies III-V and I-V and wave latency V, presented statistical significant differences in the evaluation and reevaluation. The variables representing the receiving of otoacoustic emissions in the transient form, at the frequency of 3 kilohertz and in the form distortion product in the frequencies 4, 6 and 8 kilohertz also presented significant statistical differences. Hyperbilirubinemia impairs the cochlea and the brainstem with significam alterations found in the otoacoustic emissions and in audiometry of the brainstem. An increase in the serum concentrations of bilirubin, according to a linear correlational study, alters the brainstem auditory evoked responses, which does not occur in otoacoustic emissions.
![]()
INTRODUCTION
The human auditory system provides us with the capacity to relate to our environment. For it to occur, the following items are necessary: sound conduction, sensorial reception, neural transmission, temporal cortical mechanism for discrimination and central association processes.
The auditory system, made up of anatomical elements such as ligaments, tendons, muscles, ossicles, sensorial cells and neurons, can suffer interference in its function, leading to abnormalities of the structures involved in the hearing process.
A common cause that compromises the hearing of the newborn is hyperbilirubinemia. It is normally detected in baby nurseries and is clinically manifested by jaundice. The main severe complication is “kernicterus” which can result in developmental sequels, neurological sequels and even death1, 2, in addition to deafness3, 4. The first cases of kernicterus were described in term newborns with Rhesus factor incompatibility in 1945, by Docter5.
Hyperbilirubinemia can produce toxic effects over the basal ganglia as well as in the central auditory pathways and result in deafness6.
Some authors emphasized the importance of hearing loss early diagnosis. Hyperbilirubinemia damages the inner ear and the central nervous system. Thanks to the appropriate therapy - phototherapy and/or exsanguineous transfusion, jaundice tends to respond well, causing reduction of serum bilirubin and recovery of the organs involved7. It is important to perform electrophysiologic assessment of the auditory pathways and serum concentration dosages of bilirubin in the newborn. This investigation should be performed even in conditions of low bilirubin concentrations, since even under such conditions, there are abnormalities that can be confirmed in subsequent assessments. Hearing loss is a frequent damage of hyperbilirubinemia and it is sometimes the only sign of kernicterus 3, 8.
Bilirubin, a substance produced by hemoglobin metabolism, is found in the blood circulation in physiological conditions and with no effect to the auditory system. Under specific conditions, this substance has high serum levels, sometimes greater than 10 mg/dl. The “Joint Committee on Infant Hearing”9, in 1994, referred that hyperbilirubinemia, in levels that require exsanguineous transfusion, is a risk factor for hearing loss, requiring proper assessment of the newborn.
The conduction of periodical control is important to detect the repercussion of bilirubin toxicity in the inner ear and auditory pathways10, 11.
Once the bilirubin penetrates the blood-brain barrier, it can damage the central nervous system, the kernicterus, and affect also the peripheral nerves. This affection normally leads to hearing impairment, and it may affect the following anatomical regions: cochlea, brainstem and cerebral cortex. The hearing impairment can be temporary or permanent. In newborns with jaundice there may be a deposit of bilirubin in different organs of the body, especially the brainstem, the cerebellum and the cortex.5, 12
Hyperbilirubinemia is clinically expressed as jaundice. The classification of jaundice by zones and levels of bilirubin, follows Kramer’s zones. In zone I, there is impairment of face and neck and bilirubin level is between 1 and 3 mg/dl. In zone II, there is impairment of the chest down to the navel level, and bilirubin level is between 3 and 6 mg/dl. In zone III there is impairment of the upper limbs, up to the elbow, and lower limbs up to the knee and bilirubin level ranges from 6 to 9 mg/dl. In zone IV, there is upper limb impairment up to the wrists and lower limb impairment down to the ankles, and bilirubin level ranges from 9 to 12 mg/dl. In zone V, there is impairment of hands and feet and dosage over 12 mg/dl12.
Stimulated with sound energy from the cochlea, a wave is formed and passes through the labyrinthic liquids, moving the basilar membrane and causing displacement of outer hair cells (OHC) towards the tectorial membrane. These two membranes present different phases, promoting ionic abnormalities and causing depolarization of OHC. There is an active movement modifying the length of the OHC, which amplifies the movement of the basilar membrane, which is coupled to them both. The components of this movement can be part of the formation of otoacoustic emissions. OHC are cochlear amplifiers, controlling the sensitivity of the cochlea13, 14, 15.
Indirect bilirubin is a toxic substance for in vivo and in vitro cells at least in term babies with no risk factor16.
Auditory abnormalities, such as reduction of hearing in high frequencies, damaging the cochlea and the brainstem, are detected in 33% of the cases. In the peak of physiological jaundice, 9% of the healthy newborns suffer severe hyperbilirubinemia with 15mg/dl level, whereas preterm low birth weight babies, if healthy, have an incidence of neonatal jaundice, with bilirubin values over 15mh/dl, of approximately 28% of the cases17.
Hyperbilirubinemia is one of the most significant causes affecting the newborns, since it crosses the blood-brain barrier18.
The effect of hyperbilirubinemia in neuronal excitability is transient, as shown by in vivo, in vitro and newborn studies19, 20. In studies conducted in pigs, there is no increase in enolase, which is a glycolytic enzyme and neuronal lesion marker21.
The recording of brainstem evoked auditory responses (ABR) can be used to assess the auditory pathways at any age. The younger the newborn, the greater the wave latencies. Wave I latency decreases up to the 11th week of life and wave V latency, up to 2 years22.
Nakamura4 et al., in 1985, found the presence of waveforms in six icteric babies after the appropriate therapy, and noticed only a delay in conduction time of interpeak latency I-V, corresponding to an incomplete reversion.
Tan23 et al., in 1992, studied 30 hyperbilirubinemia newborns with dosages over 15 mg/dl and 30 newborns in the control group and conducted ABR in all of them. They were all submitted to phototherapy for 24 hours. The bilirubin dosage was checked in 6 and 12 periods and it reached 11 mg/dl. ABR values were statistically different in newborns with hyperbilirubinemia when compared to those in the control group for wave V latency and interpeak latency III-V and I-V. The improvement in ABR continued to be similar to the control group, but the authors did not manage to determine the critical level of bilirubin that modify the brainstem auditory evoked responses.
In a longitudinal study, indirect bilirubin level was initially greater than 22 mg/dl, and then dropped to nearly 15mg/dl after exsanguineous transfusion, which confirmed the recovery of normal ABR tracings10.
The stimulus conduction time up to the brainstem is increased in hyperbilirubinemia newborns. At bilirubin levels greater than 28 mg/dl all auditory brainstem evoked responses are abnormal. When the substance is at levels of 24 mg/dl to 28 mg/dl, there is an incidence of 55% of abnormalities in the ABR 24.
The most common ABR abnormalities in newborns with bilirubin levels greater than 15 mg/dl are high thresholds of wave V, absence of all waves at 90dbHL, prolonged latency of various waves and maintenance of ABR abnormalities25.
Considering what was presented here, we came up with the following investigation question: At what extent and how does hyperbilirubinemia affect the hearing system of the newborns assessed by otoacoustic emissions and auditory brainstem evoked responses?
The following hypothesis was developed for this study: there is a correlation between newborn hyperbilirubinemia and auditory system affection detected by otoacoustic emissions and auditory brainstem evoked responses.
The general objective was to study the auditory system up to the brainstem in icteric term newborns appropriate to gestational age.
The specific objectives of the study were:
1. To determine the abnormalities of the cochlear function in term icteric newborns appropriate for gestational age using otoacoustic emissions;
2. To determine brainstem abnormalities in term icteric newborns appropriate for gestational age using auditory brainstem evoked responses;
3. To compare the bilirubin serum levels to abnormalities detected in the hearing system.
MATERIAL AND METHOD
Our study design was descriptive-exploratory, longitudinal and correlational. The sample unit was the subject and it included 64 newborns. Thirty-two of them were part of the control group (GC) and 32 formed the icteric group (GI). Out of the subjects in the GI, 21 were male and 11 were female subjects. Thirty-one newborns were Caucasian and one was Black. Activities were conducted in 14 hospitals, being that five of them were university hospitals, located in 12 cities of the states of Minas Gerais and Sao Paulo. The tests were conducted in a room of the nursery of public or private hospitals in an environment with low background noise.
The newborns in the GC were assessed as of the 3rd day of life and the inclusion criterion was serum bilirubin concentration below 10 mg/dl. As to GI, the auditory system was assessed when bilirubin serum concentration was at its peak, which was called initial icteric group (GII). This group was reassessed when bilirubin serum concentrations normalized and it formed the final icteric group (GIF). No newborns had any other diseases except for hyperbilirubinemia and met the inclusion and exclusion criteria.
All newborns were submitted to the same hearing tests. Regardless of the group, they underwent auditory system assessments and they were divided as follows: 1. Pilot group (GP) - 12 newborns; 2. Control group (GC) - 32 newborns; 3. Icteric group (GI) - 32 newborns, submitted to longitudinal study - Initial icteric group (GII), assessed between the first and 24th day after birth, and final icteric group (GIF), assessed between the 26th and 73rd day after birth.
Assessment of the newborns in groups GP, GC and GII were conducted once between birth and the 24th day of life. GIF consisted of the same newborns from GII who were reassessed once the bilirubin serum levels were normalized. Newborns in the GI group were excluded if there were not complete test data.
The data collected, comprising clinical history, physical examination, lab tests and results from the auditory assessments, were collected from all newborns as part of an Assessment Form.
Auditory function assessment, emphasizing outer hair cells and brainstem, was conducted by means of otoacoustic emissions and ABR. We also performed meatoscopy and immittanciometry to check status of external and middle ear, respectively.
The present study was conducted with our own equipment, as listed below, including the specific techniques and interpretation analyses: to assess otoacoustic emissions, “Celesta 503-Cochlear Emissions Analyzer” (Version 3.xx), manufactured by Madsen Electronics Norway. This test, as well as the others, was conducted by the same professional. We conducted transient otoacoustic emissions (TOAE) and distortion product otoacoustic emissions (DPOAE). The device was coupled to a portable microcomputer, brand Extensa, 166 MHz and 16 MB RAM, for storage and printing.
We used the following parameter for TOAE: stimulus suppression period: 3.00 ms; Duration of stimulus: 80ms; Acquisition mode: non-linear; Stimulus polarity: condensed; Level of stimulus: 80dB SPL; scheduled stimuli: 3,000.
As to DPOAE, the parameters were: f2/f1: 1.22; f0: specific study frequency; f1: first primary frequency and below the specific frequency; f2: second primary frequency and above the specific frequency; 2f1-f2: below f1; sound intensity: 70dB SPL; total of stimuli: 3,000.
We studied total responses (T) for TOAE and the responses of frequencies 1, 2, and 3 kHz expressed in total acoustic energy. They were grouped together with the absolute value of reproducibility for the subsequent analysis.
As to DPOAE, we studied frequencies 0.75, 1, 2, 3, 4, 6, and 8 kHz. By the end of the test, we recorded the responses for each frequency for later printing purposes.
The device used for auditory brainstem evoked responses was the portable audiometer “GSI 55 ABR Screener”, manufactured by “Welch Allyn Company”. The device had four contact electrodes, being one for reference, one neutral, and one for each earlobe. The tests were performed by the same professional, as mentioned before.
The parameters for recording were: a) monaural stimuli and recording; c) polarity: alternating; c) number of stimuli: 1,024; d) Duration of analysis: 12 ms; e) Sound intensity: 70dB HL; f) Filter between 150 and 2,000Hz; g) The stimulus used was a 100ms click; h) division by 100nV; i) we used contact electrodes.
We studied the latency of waves I, III and V. We defined latency as the interval between the beginning of the stimulus and the onset of the corresponding wave. The interpeak intervals I-III. I-V and III-V are the differences between the onset of one wave and the other. The amplitude of waves I, III and V is the distance between the largest positive deflection and the main negative deflection posterior to it. The latency and interpeak interval measures were obtained in milliseconds. Wave amplitude was obtained in nanovolt (nV).
Reference values for interpeak variables, wave amplitude and wave latency, previously established as normal range, were defined from the control group.
The values of total bilirubin were defined as parameters and in mg/dl (Burtis; Ashwood, 1998):
1. umbilical cord dosage: below 2 mg/dl;
2. 0 to 1st day: 2.0 to 6.0 mg/dl;
3. 1st to 2nd day: 6.0 to 10 mg/dl;
4. 3rd to 5th day: 1.5 to 12 mg/dl;
5. adult: 0.3 to 1.2 mg/dl.
The values were used for guidance. Out of the total bilirubin, the main percentage is formed of indirect bilirubin of free circulation12.
The present study complies with the Resolution 196/96 that addresses research studies in human beings. The project was approved by the Research Ethics Committee of the Medical School, University of São Paulo.
The variables were presented by mean, standard deviation (SD) and median. We employed the following statistical tests: t Student’s and Pearson’s linear correlation (r). We adopted the significance level of 0.05 (a = 5%). Descriptive values (P) below this point were considered statistically significant.
RESULTS
The results of the present study are displayed in figures. The values found in the Assessment Form of each newborn were analyzed considering GC, GII and GIF.
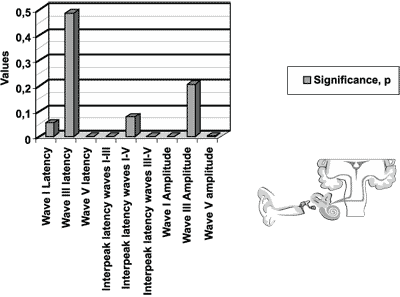
The significance level of the measures for the ABR test, presented in Figure 1, represents the following variables: latency of waves I, III and V; interpeak intervals I-III. I-V and III-V; amplitude of waves I, III and V, for newborns in the initial icteric group and final icteric group and the comparison between them.
We found statistically significant differences for variables of wave V latency, interpeak latencies I-V and III-V, and amplitudes of waves I and V.
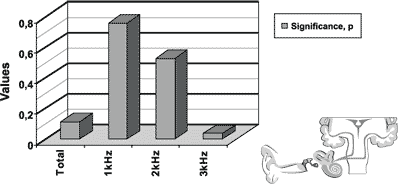
The results of transient otoacoustic emissions for the initial and final icteric groups are presented in Figure 2.
Wee found statistically significant difference for the frequency of 3kHz.
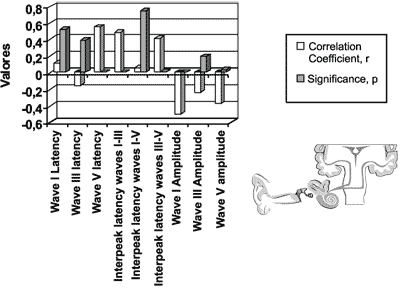
The coefficient of linear correlation and the significance level between dosage of total bilirubin level and ABR measures (latency of waves I, III and V; interpeak intervals I-III, I-V and III-V; amplitude of waves I, III and V), in the initial icteric group are presented in Figure 4.
We found statistically significant differences for variables latency of wave V, interpeak intervals I-V and I-III and amplitude of waves I and V.
We did not find statistically significant differences for otoacoustic emissions in the correlational study.
Figure 1. Results of auditory brainstem evoked potentials for the initial icteric group and the final icteric group by applying t Student test for related samples.
Figure 2. Results of transient otoacoustic emissions for the initial icteric group and the final icteric group by applying t Student test for related samples.
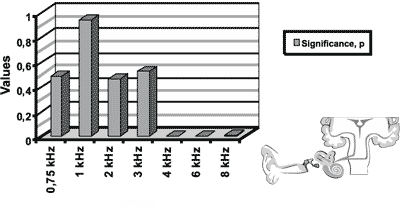
Figure 3. Results of distortion product otoacoustic emissions for the initial icteric group and the final icteric group by applying t Student test for related samples.
Figure 4. Auditory brainstem evoked potentials for the initial icteric group and the final icteric group by applying Pearson’s correlation coefficient.
DISCUSSION
The processing of acoustic information at the age of 2y and 6m is similar to that in adults, although wave V is still under maturation at the age of 2 years22. Wave I latency reduces progressively up to the 3rd week of life. The impairment of the auditory system has a direct correlation with serum concentration of indirect bilirubin 26.
The importance of this study is highlighted by the possibility of sequels caused by hyperbilirubinemia in newborns8, 18, 27. Another issue is the fact that hyperbilirubinemia is part of the risk factor list by the “Joint Committee on Infant Hearing”, of the Academy of Pediatrics9, issued in 1994.
Hearing loss can be a result of the toxic effect of bilirubin on the cochlea and the basal ganglia6, 10, 11.
Therefore, we will analyze separately the effects of hyperbilirubinemia over the cochlear function using otoacoustic emissions and over the auditory basal ganglia using auditory brainstem evoked responses.
AUDITORY FUNCTION OF BRAINSTEM NUCLEI
Since the first cases of kernicterus presented by Docter5, in 1945, the sequels of neurological compromise and death have been described. Other studies1, 2, 6 showed that hearing loss was very common among hyperbilirubinemia newborns. In our study, we found absence of ABR tracings in two newborns, in the reassessment, one at 70dB HL and the other at 55 dB HL.
The depending variables analyzed through ABR such as latency of wave V, interpeak intervals III-V, I-V and amplitude of waves I and V, when compared between the groups GII and GIF, presented statistically significant differences.
The amplitude of waves I and V presented statistically significant differences when groups GII and GIF were compared. The correlational analysis between them was negative and presented statistically significant result6, 21, 28.
Wave V latency and its standard deviation for the initial and final icteric groups was 6.72 ± 0.28 and 6.24 ± 0.32 ms with statistically significance. We found positive correlation for ABR abnormalities and bilirubin serum levels26.
The values found by Rodrigues Fernández et al.11 in different diseases, in a total of 40 children, were 6.55 ± 0.43 and 6.63 ± 0.43 for the right and left ears, respectively, which are values below the ones we detected, which were 6.72 ± 0.28 ms.
Some studies have shown that wave latency in ABR decreases as a result of the maturation process after birth, presenting negative correlation 22.
Significant ABR abnormalities in the interpeak intervals I-III and I-V were found in the studies by Tan et al.23 in 1992, in newborns with serum bilirubin dosages greater than 15 mg/dl. We found statistically significant differences in the icteric group for interpeak intervals I-V and II-V and positive linear correlation6, 10, 24.
There was a significant correlation between the values of wave V latency, interpeak intervals III-V and I-V, and amplitude of waves I and V, and bilirubin serum concentrations. These results were confirmed when we defined a cutoff point in the value of serum bilirubin at 18 mg/dl, creating two subgroups and all results mentioned before were statistically significant, confirming the previous findings. Since we did not create other cutoff points, we do not know which would be the minimum serum concentration that still had statistically significant correlation.
The correlation between bilirubin serum level and wave V latency or interpeak intervals III-V and I-V was positive. The variable-dependent increase in abnormal auditory responses occurred as a result of the increase in variable-independent bilirubin serum concentration. The variable-dependent amplitude of waves I and V presented negative correlation, since the increase in serum bilirubin reduced the wave amplitude.
When the newborn babies presented high rates of serum bilirubin, they also present many ABR abnormalities; after treatment, as the substance is reduced, there is the consequent normalization of ABR tracings23. The opposite happened, according to a study by Funato26 et al. in 1994, in 37 newborns which showed positive correlation of ABR abnormalities and increase ion serum concentration of bilirubin.
Based on the statistically significant results for interpeak intervals I-V and III-V and wave V amplitude, we confirmed the involvement and the impairment of the brainstem3, 6, 17.
We detected a significant improvement in ABR tracings in hyperbilirubinemia newborns after its normalization, confirmed by the assessment of the final icteric group6, 9, 24.
Studies by Tan23 et al., in 1992 did not detect the critical value of bilirubin to produce ABR abnormalities either.
COCHLEAR FUNCTION
In cases of hyperbilirubinemia, according to Chisin; Sohmer3, the cochlear function assessed by means of otoacoustic emissions is abnormal, showing absence of responses. According to Anand; Gupta17 and Funato26 et al., the cochlear impairment is present in 33% of the hyperbilirubinemia newborns, causing prolongation of wave I latency and reduction of its amplitude, leading us to the conclusion that hyperbilirubinemia would compromise the cochlea.
Cochlear impairment, selectively to the outer hair cells, was evidenced through TOAE and DPOAE, but the correlational study was not statistically significant.
In the analysis of the results of otoacoustic emissions for the initial and final icteric groups, we found statistically significant differences in the transient test in the frequency of 3kHz. As to distortion product, we found statistically significant differences for frequencies of 4, 6 and 8kHz.
We did not detect correlation between the responses in otoacoustic emissions and bilirubin serum concentrations.
There are no literature reports studying otoacoustic emissions and hyperbilirubinemia. Based on the published studies, we would not learn about the frequencies using electrocochleography, behavioral audiometry and brainstem auditory evoked responses applied to the newborns. Otoacoustic emissions have come to bridge this gap, telling us about cochlear functioning and presenting responses by frequencies. ABR does not analyze high frequencies, normally above 4kHz. The click is not a specific stimulus and normally stimulates the cochlea, but mainly in frequencies between 2 and 4kHz.
The impairment of high frequencies collected by otoacoustic emissions confirmed the findings by Anand; Gupta17 in 1993, who reported the same auditory impairment in hyperbilirubinemia newborns.
We detected only one newborn in the study group who presented definite cochlear pathology, confirmed by otoacoustic emissions.
The assessment of the auditory system in high risk newborns, especially in hyperbilirubinemia patients, is extremely important13, 14, 15.
The energy collected by otoacoustic emissions from each specific regions of frequencies in the initial icteric group was smaller than those collected in the final icteric group.
We did not find statistically significant difference in linear correlation coefficients between bilirubin dosage and responses to otoacoustic emissions.
CONCLUSIONS
As a result of the effects of hyperbilirubinemia in the auditory system, we concluded the following by studying the differences between results from the initial and final icteric groups:
Cochlear function - There was a clear impairment of cochlear function in hyperbilirubinemia newborns, as seen below:
1. In abnormalities found in TOAE in the region of frequency 3 kHz;
2. In abnormalities found in DPOAE in the region of frequencies 4, 6 and 8kHz.
Brainstem auditory function – There was a clear impairment of brainstem auditory function in hyperbilirubinemia newborns, as seen below:
3. In abnormalities found in the brainstem, assessed by auditory brainstem evoked responses, observing prolongation of wave V latency.
4. In abnormalities found in the brainstem, assessed by auditory brainstem evoked responses, observing increase in interpeak intervals III-V and I-V.
5. In abnormalities found in the brainstem, assessed by auditory brainstem evoked responses, observing reduction of amplitude of waves I and V.
Serum levels of bilirubin
6. There was a positive correlation between bilirubin serum concentration and affection of auditory system in the ABR for the variables wave V latency and interpeak intervals I-V and III-V.
7. There was a negative correlation between bilirubin serum concentration and affection of auditory system in the ABR for amplitude of waves I and V.
8. There was no correlation between bilirubin serum concentration and affection of auditory system in otoacoustic emissions and different frequencies.
REFERENCES
1. Nilsen ST, Finne PH, Bergsj P, Stamnes O. Males with neonatal hyperbilirubinemia examined at 18 years of age. Acta Paediatr Scand 1984;73:176-80.
2. Perlstein MA. The late clinical syndrome of posticteric encephalopathy. Pediatr Clin Noth Am 1960;7:665-87.
3. Chisin R, Perlman M, Sohmer H. Cochlear and brainstem responses in hearing loss following neonatal hyperbilirubinemia. Ann Otol Rhinol Laryngol 1979;88:352-7.
4. Nakamura H, Takada S, Shimabuku R, Matsuo M, Matsuo T, Negishi H. Auditory nerve and brainstem evoked responses in newborn infants with hyperbilirubinemia. Pediatrics 1985;75:703-8.
5. Docter JM. Kernicterus: neurological sequelae of erythroblastosis fetalis. J Pediatr 1945;27:327-34.
6. Funato M, Teraoka S, Tamai H, Shimida S. Follow-up study of auditory brainstem responses in hyperbilirubinemic newborns treated with exchange transfusion. Acta Paediatr Jpn 1996;38:17-21.
7. Riko K, Hyde ML, Alberti PW. Hearing loss in early infancy: incidence, detection and assessment. Laryngoscope 1985;95:137-45.
8. Newman TB, Vohr BR, Oh W, Cashore WJ. Neoanatal jaundice and brain-stem auditory responses. J Pediatr 1991;118, 653.
9. Joint Committee on Infant Hearing. Position statement 1994. ASHA 1994;36:38-41.
10. Deorari AK, Singh M, Ahuja GK, Bisht MS, Verma A, Paul VK, Tandon DK. One year outcome of babies with severe neonatal hyperbilirubinemia and reversible abnormality in brainstem auditory evoked responses. Indian Pediatr 1994;31:915-21.
11. Rodríguez Fernández MP, Guillen Morales A, Gonzáles Delgado A. Evalución de la audición em lactantes com alto riesgo con potenciales evocados auditivos del tallo cerebral. An Otorinolaringol Mex 1995;40:127-30.
12. Vaz FAC, Ramos JLA, Araujo MCK. Icterícia do recém-nascido. In: Marcodes E, ed. Pediatria Básica. São Paulo: Sarvier; 1994;1:395-407.
13. Brownell WE, Bader CR, Bertrand D, Ribaupierre Y. Evoked mechanical response of isolated cochlear outer hair cells. Science 1985;227:194-6.
14. Gattaz G, Cerruti VQ. O uso do registro de emissões otoacústicas evocadas para triagem auditiva em neonatos de risco para deficiência auditiva. Rev. Paulista de Pediatria 1994;12:291-4.
15. Pialarissi PR, Gattaz G. Emissões otoacústicas, conceitos básicos e aplicações clínicas. Arquivos da Fundação Otorrinolaringologia 1997;1:41-3.
16. Scheidt PC, Mellitus D, Hardy JB, Drage JS, Boggs TR. Toxicity to bilirubin in neonates: Infant development during first year in relation to maximum neonatal serum bilirubin concentration. J Pediatr 1977;91:292-7.
17. Anand NK, Gupta AK. What constitutes “safe” level of bilirubin concentration in preterm and full term infants? Indian J Pediatr 1993;60:475-83.
18. Bhandari V, Narang A, Mann SB, Raghunathan M, Bhakaoo ON. Brainstem electric, response audiometry in neonates with hyperbilirubinemia. Indian J Pediatr 1993;60:409-13.
19. Chin KC, Taylor MJ, Perlman M. Improvement in auditory and visual evoked potentials in jaundiced preterm infants after exchange transfusion. Arch Dis Child 1985;60:714-7.
20. Nwaesei CG, Van Aerde J, Boyden M, Perlman M. Changes in auditory brainstem responses in hyperbilirubinemic infants before and after exchange transfusion. Pediatrics 1984;74:800-3.
21. Hansen TWR, Cashore WJ, Oh W. Changes in piglet auditory brainstem response amplitudes without increases in serum or cerebrospinal fluid neuron-specific enolase. Pediatr Res 1992;32:524-9.
22. Gorga M, Kaminski J, Beauchaine K, Jesteadt W, Neely S. Auditory brainsteam responses from children three months to three years of age: normal patterns of response II. J Speech Hear Res 1989;32:281-8.
23. Tan KL, Skurr BA, Yip YY. Phototherapy and the brain-stem auditory evoked response in neonatal hyperbilirubinemia. J Pediatr 1992;120:306-8.
24. Gupta AK, Mann SB. Is auditory brainsteam response a bilirubin neurotoxicity marker? Am J Otolaryngol 1998;19:232-6.
25. Agrawal VK, Shukla R, Misra PK, Kapoor RK, Malik GK. Brainstem auditory evoked response in newborns with hyperbilirubinemia. Indian Pediatr 1998;35:513-8.
26. Funato M, Tamai H, Shimada S, Nakamura H. Vigintiphobia, unbound bilirubin, and auditory brainstem responses. Pediatrics 1994;93:50-3.
27. Garza Morales S, Poblano A, Robledo Galván A, Fernández Carrocera LA. Potenciales provocados auditivos en niños com riesgo neonatal de hipoacusia. Pan Am J Public Health 1997;1:119-24.
28. Lenhardt ML, Mcartor R, Bryant B. Effects of neonatal hyperbilirubinemia on the brainstem electric response. J Pediatr 1984;104:281-4.
29. Elssman S, Matkin N, Sabo M. Early identification of congenital sensorioneural hearing impairment. Hear J 1987;40:13-7.
30. Wennberg RP, Ahlfors CE, Bickers RG, Mcmurtry CA, Shetter JL. Abnormal auditory brainstem response in newborn infant with hyperbilirubinemia: improvement with exchange transfusion. J Pediatr 1982;100:624-6.
1 Professor, Ph.D., Medical School of Itajubá.
2 Professor, Ph.D., Pontifical Catholic University of São Paulo.
3 Professor, Ph.D., Department of Otorhinolaryngology, Medical School of the University of São Paulo.
4 Professor, Master in Scientific Methodology, Nursing School of Itajubá.
Partial excerpt of the doctorate dissertation submitted to the Discipline of Otorhinolaryngology, Medical School of the University of São Paulo, in 2000.
Study conducted at Hospital Odontomed, in Itajubá and in 13 other hospitals in the South of the state of Minas Gerais and Vale do Paraíba, state of São Paulo.
Address correspondence to: Hospital Odontomed – Rua Major Belo Lisboa, no 88
Centro Itajubá-MG 37500-000
Tel/Fax (55 35) 3621-2000 – E-mail: Fsalesdr@sulminas.com.br
Article submitted on August 31, 2001. Article accepted on September 05, 2002