Year: 2002 Vol. 68 Ed. 6 - (1º)
Artigos Originais
Pages: 781 to 787
The expression of VCAM-1 in patients with nasal polyposis
Author(s):
Richard L. Voegels1,
Patricia Santoro2,
Ossamu Butugan3,
Aroldo Miniti4.
Keywords: nasal polyposis, allergy, adhesion molecules, VCAM-1
Abstract:
Nasal polyposis (NP) is a chronic inflammatory disease of the nasal mucosa. The etiology and formation of NP are still not elucidated and Nave been debated for many years. Aim: The objective of the present study was to investigate the role of the expression of VCAM-1 in the endothelium of the group of patients: allergic with NP, non-allergic with NP and a control group. Study design: Clinical prospective. Material and method: Thirty-nine patients with NP were selected, 13 of them allergic and 26 non- allergic. A control group of 11 individuals was also studied. The expression of VCAM-1 was investigated with immunohistochemistry of frozen tissue. There was a higher incidence of NP after the fourth decade of life and among men. The clinical symptoms were similar in both groups of patients with nasal polyposis and characterized by nasal obstruction and anosmia. Conclusion: The expression of VCAM-1 was significant in both groups of patients with nasal polyposis, suggesting that this adhesion molecule is probably involved in the intense migration of eosinophils that occurs during nasal polyp formation.
![]()
INTRODUCTION
Nasal polyposis (NP) is a chronic inflammatory disease of the nasal mucosa that affects more than 0.5% of the population1, 2. Most of the times, the patients present intense eosinophilic infiltrate. The mechanisms and the significance of this infiltrate are still reason for controversy, since there are innumerous theories described in the literature3-6.
Many authors defined NP as a result of an allergic IgE mediated process, and the marked eosinophilia would be a result from this process7, 8, 9. Other authors believe that the eosinophilic migration presented in NP would not be related with allergy, but rather a result of a different immune mechanism10, 11.
In order to better understand the eosinophilic migration present in most patients with NP and the immune mechanisms involved in its genesis, we created groups of patients to study nasal polyposis: allergic and non-allergic patients to be compared to a control group. The purpose of the present study was to investigate the role of the expression on the vascular endothelium of the adhesion molecule VCAM-1 in the eosinophilic migration of patients with NP.
MATERIAL AND METHOD
We prospectively studied 39 patients with nasal polyposis seen and operated on during the 2nd semester of 1997 at the Division of Clinical Otorhinolaryngology, Hospital das Clinicas, Medical School, University of São Paulo. The following were the inclusion and exclusion criteria used in the study:
• Inclusion criteria: We included only patients that presented diagnosis of bilateral nasal polyposis to be submitted to surgical treatment;
• Exclusion criteria: We excluded patients with single polyp (antrochoanal, sphenochoanal) or other diseases related to nasal polyposis, such as cystic fibrosis, primary ciliary dyskenesia and fungal sinusitis. Finally, we excluded those that could not be classified as allergic or non-allergic.
The 39 selected patients were divided into two groups:
1. allergic group (13 patients)
- history of allergic rhinitis (nasal pruritus, sneezes, rhinorrhea, nasal obstruction)
- positive hypersensitivity skin test
2. non-allergic group (26 patients)
- absence of history of allergic rhinitis
- negative hypersensitivity skin test
Control group: It comprised 11 volunteer adults, no past history of nasal symptomatology and/or allergic symptoms of any nature. The nasal fossa examination, with rigid endoscope, was normal. In this group, 7 subjects (63.3%) were male and 4 (36.4%) were female. The mean age was 29.8 years (5.3 years, 25-42 years, mean, standard deviation).
All patients were assessed one week before the surgery and they were asked to score their symptoms from 1 to 10: headache, nasal obstruction, hyposmia, purulent secretion, coriza and cough. We considered as score 1 the state without symptom and score 10 as a very severe symptom. We also collected blood for IgE and eosinophils, in addition to performing allergic skin test.
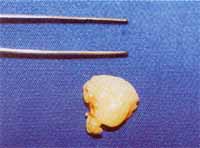
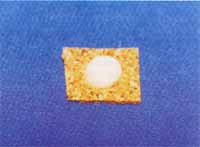
The collection of fragments of nasal polyps was made during the surgical act under general anesthesia (Figure 1). We separated two fragments of the polyp of each patient, one was submitted to staining with hematoxylin and eosin whereas the other was placed over a piece of cork measuring 1.0 cm x 1.0cm x 1.0mm thick. It was fully recovered by CRYO-GEL® (Instrumedics Inc., NJ, USA) avoiding the formation of air bubbles. We then immersed the fragment recovered with CRYO-GEL® in a mist of liquid hydrogen up to solid state of the whole mass (approximately 10 minutes) (Figure 2). The fragment was wrapped in aluminum foil and properly identified. All procedures were conducted in the operating room and then immediately transported in a thermos with liquid nitrogen to the freezer at -70oC where the material was stored for the immunohistochemical reaction10.
The collection of fragments of normal mucosa of the control group was made with Grünwald clamps. We removed mucosa of the middle concha under observation with rigid endoscope at 4.0mm and 30o. The mucosa of the patient was submitted to the same process described for the polyps.
Through the immunohistochemical reaction we investigated antigens in the nasal polyp with primary mice monoclonal antibody against human VCAM-1 (CD106), clone 1.4C3, IgG, kappa (DAKO M 7106, Denmark), diluted at 1:50, through the complex alkaline phosphatase/alkaline anti-phosphatase (APAAP complex).
The fragment was frozen by chock with liquid nitrogen and stored at -70oC to better preserve the antigen, as opposed to tissues fixated in formol and included in paraffin. The frozen fragments were sectioned at 3mm thick sections using a cryostat and were adhered to treated slides with SILANE A174 adhesive (Pharmacia, USA). Each slide received 4 sections, one for negative control reaction and three for dilution of the primary monoclonal antibody. The sections prepared as such were stored at 4o C for one night (fourteen hours)10 and then fixated in acetone (21605-MERCK) at 4°C for 10 minutes. After that, they were dried at room temperature for 10 minutes, wrapped in aluminum foil and PVC film and stored at -70oC up to the processing of the reactions.
The immunoenzymatic method used was APAAP complex10. Tissue antigen was exposed initially to the specific monoclonal antibody, later to a secondary antibody not labeled and directed against primary murine immunoglobulin, and finally to a murine APAAP complex that also binds to secondary antibody and reacts with a specific enzymatic substrate. The main steps of the procedures were the following:
• primary incubation: the first section (negative control) and the two subsequent ones with the primary monoclonal antibody in two successive dilutions.
• secondary incubation of all sections with Rabbit-anti-mouse; RAM (Rabbit-Anti-Mouse Immunoglobulins code nº Z 259, lot nº 071, DAKO, Denmark), in1:100 dilution.
• tertiary incubation of all sections with the complex APAAP (APAAP Mouse Monoclonal code nº D 651, lot no 023, DAKO, Denmark) (Figure 9), in 1:100 dilution.
• final incubation with the substrate associated with levamisole (to block endogenous alkaline phosphatase)10.
• The comparison between the groups was made using parametric and non-parametric statistical tests taking into consideration the nature of data and variability of the measures made. We used statistical tests, all of them with significance level at 5%12, 13.
Table 1. Concentration of eosinophils and immunoglobulin found in the studied groups
NOTE: SD - standard deviation
* values in percentage of the total of leukocytes
** values expressed in international units/ milliliter (IU/mL)
*** values expressed in milligram/ deciliter (mg/dL)
Values that were statistically significant are marked (p£ 0.05)
Statistical test: Kruskal-Wallis test (eosinophils and IgE)
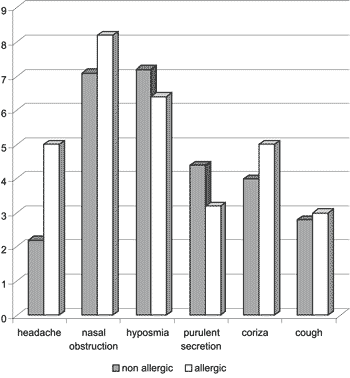
Graph 1. Assessment of clinical manifestations by the patients
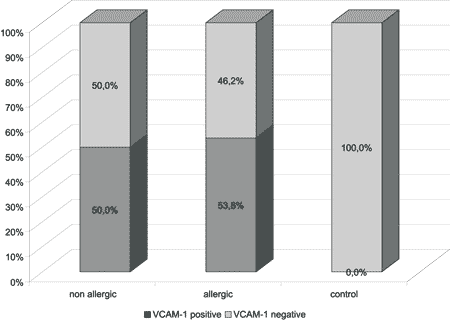
Graph 2. Distribution of patients after VCAM-1 positivity.
RESULTS
The age range of patients varied from 16 years to 88 years. The mean age was 42.6 years (16.9 years, 16-88 years, mean, standard deviation).
As to gender, 22 patients (56.4%) were male and 17 (43.6%) were female subjects.
Upon the comparison of the arithmetic scores of clinical pictures of the allergic and non-allergic groups, we did not observe statistically significant difference between the groups in symptoms (Graph 1).
The serum levels of eosinophil and immunoglubulin are represented in Table 1. The eosinophilic parameters and IgE in the group of allergic patients presented levels significantly greater (p<0.001) than the other groups.
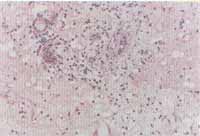
The histology analysis using hematoxylin and eosin staining showed intense eosinophilic infiltrate in 37 (94.8%) of the 39 patients with NP (Figures 3 and 4). In two tests, we did not visualize significant tissue eosinophilia, being that both belonged to the non-allergic group.
The results of the immunohistochemical analysis are exposed in Graph 2 and they were expressed as positive (yes) or negative (no) (Figures 5 and 6). There was no statistically significant difference between the two groups of NP, however, patients in the control group were classified as negative and therefore, there was a statistically significant difference from the others (p<0.001).
Figure 1. Nasal polyp right after surgical removal.
Figure 2. Upper view of the polyp recovered in CRYO-GELâ and frozen by liquid nitrogen mist.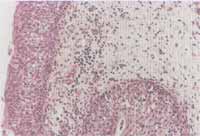
Figures 3 and 4. Intense eosinophilic infiltrate through the whole tissue (nasal polyp) (optic microscopy - H&E x100 and x200).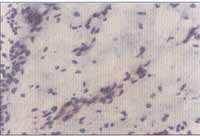
Figures 5 and 6. Immunohistochemical reaction demonstrating the expression of VCAM-1 in the vascular endothelium of the nasal polyp (optic microscopy - x200).
DISCUSSION
The exact pathophysiology of the NP is still unknown3, 4, 6, 14-20. The patients present a complex and intense inflammatory infiltrate with the presence of innumerous chemical mediators9, 21.
Among the patients with NP, there is predominance of subjects over the age of 30 years, representing nearly 75% (29/39) of the total. Such data confirm those reported in the literature. Settipane and Chafee22, in a revision of 149 cases, found 122 (82%) aged 30 years or over. Similar data were reported by Jamal; Marant23 and Granström et al.8, suggesting high prevalence of the disease after the third decade of life.
We did not find statistically significant difference between age of patients in the two studied groups - allergic and non-allergic patients. However, whereas the mean age in the allergic group was 37.3 years, the mean age in the second group was 45.3 years.
It is interesting to observe that, similarly to the literature, we found few cases of affection in the first two decades of life, suggesting that except for cystic fibrosis, NP is rarely detected in this age range.
Out of the total of 39 patients with NP, 22 (56.4%) were male subjects. The data confirm those reported in the literature, stating predominance of male patients8, 23, 24.
The patients with NP normally present simultaneous chronic sinusitis, and the clinical picture is frequently characterized by headache, nasal obstruction, hyposmia, purulent secretion, running nose and cough.
Even though some authors suggested that the presence of allergy associated with NP could exacerbate the symptomatology of patients25, in our sample we did not observe statistically significant difference between the two groups (allergic or non-allergic) concerning clinical presentation. In both groups, the main symptoms were nasal obstruction and hyposmia. The group of allergic patients presented worse scores for headache, nasal obstruction, running nose and cough. However, there was no statistically significant difference for any symptom.
The role of atopy in the pathophysiology of NP is still very controversial. Many authors believe that allergy has an important role in the formation of polyps7-9, 26-29. However, in a recent study with cytokines, the opposite has been suggested11, 30, 31. Considering such polemical issue, we divided our patients into two groups, the allergic and non-allergic patients. The allergic patients amounted to 33.3% of the total number of patients with NP, which would correspond to incidence of atopia in the general population of a great urban center, such as São Paulo32.
Settipane et al.25 emphasized that, despite the fact that nasal polyposis is not more frequent in atopic patients, if the diseases are both present, there is exacerbation of nasal polyposis.
There is still a strong correlation between these data and serum levels of IgE. However, there was no uneven level of IgE serum level between the control group and the non-allergic patients, the IgE serum level found in allergic patients was significantly greater than the control group (p<0.001) and the non-allergic patients (p<0.001).
The adhesion molecules are proteins of the membrane that enable that two cells interact. Such fact can be modulated both by the increase in number of adhesion molecules exposed on the cell surface and by abnormal affinity between the same molecules33.
The main site of expression of adhesion molecule is the vascular endothelium, being ICAM-1 and VCAM-1 the main representatives of this group34. The interaction of these molecules, present in the endothelial cell surface, with the respective active contra-receptors on the inflammatory cell membrane, facilitates the transendothelial migration of the latter to inflammatory sites35.
In the case of patients with NP, the main inflammatory cell of the NP tissue is eosinophil 8. Over 90% of the nasal polyps present intense eosinophilia 2. In our sample, only two patients (5.1%) did not have such characteristic, being that both belonged to the non-allergic group and presented negative immunohistochemical reaction to VCAM-1.
The exact mechanism involved in the transepithelial migration of these cells in these patients has not been properly defined yet.
In order to check the expression of VCAM-1 in the nasal polyp tissue, we decided to use the method of immunohistochemistry in frozen tissue. The advantage of this method is that it enables visualization of the exact site where there is expression of VCAM-1. However, the difficulty to quantify the result, especially in the nasal polyp tissue, in which there is great variation of tissue density in polyps of different patients, is a setback. For this reason, we decided to classify the patients in two groups:
1. positive, when at least one blood vessel was positive to VCAM-1;
2. negative, when no positive vessel for VCAM-1 was visualized throughout the complete extension of the studied section.
This type of positive and negative classification has also been used by other authors36.
Symon et al.37 did not refer increase in the expression of VCAM-1 in NP tissue in any of the eight studied patients. Other authors demonstrated an increase in expression of VCAM-1 in the vascular endothelium of allergic and non-allergic patients with NP36, 38-40. They, therefore, suggested that VCAM-1 would be involved in the transepithelial migration of eosinophils to the NP tissue.
In our sample, we did not observe expression of VCAM-1 in the vascular epithelium of the normal mucosa of any patients in the control group. Conversely, in NP patients we detected the expression of VCAM-1 in more than half of them, both in the allergic and non-allergic groups. There was no statistically significant difference between the two groups. Thus, the expression of VCAM-1 was significant in both groups of patients with NP when compared to the control group.
CONCLUSION
The expression of VCAM-1 was significantly greater in patients with nasal polyposis, regardless of the presence of allergy, suggesting that the adhesion molecule is possibly involved in the intense migration of eosinophils that is detected in the disease.
REFERENCES
1. Caplin I, Haynes JT, Spahn J. Are nasal polyps an allergic phenomenon? Ann Allergy 1971;29:631-4.
2. Settipane GA, Lund VJ, Berstein JM, Tos M. Nasal polyps: epidemiology, pathogenesis and treatment. Rhode Island: Oceanside Publications; 1997. 97p.
3. Bernstein JM, Gorfien J, Noble B, Yankaskas JR. Nasal polyposis: Immunohistochemistry and bioelectrical findings (a hypothesis for the development of nasal polyps). J Allergy Clin Immunol 1997;99:165-75.
4. Davison FW. Hiperplastic sinusitis, a five year study. Ann Otol 1963;72:462-74.
5. Norlander T, Fukami M, Westrin KM, Stierna P, Carlsöö B. Formation of mucosal polyps in the nasal and maxillary sinus cavities by infection. Head Neck Surg 1995;109:522-9.
6. Tos M. The pathogenetic theories in formation of nasal polyps. Am J Rhinol 1990;4:51-56.
7. Calenoff E, Guilford T, Green J, Engelhard CS. Bacteria-specific IgE in patients with nasal polyposis. Arch Otolaryngol 1983;109:372-5.
8. Granström G, Jacobsson E, Jeppsson PH. Influence of allergy, asthma and hypertension on nasal polyposis. Acta Otolaryngol 1992;492:22-7.
9. Jacobs RL, Freda EJ, Culver WG. Primary nasal polyposis. Ann Allergy 1983;51:500-5.
10. Bachert K, Wagenmann M, Hauser U, Rudack C. IL-5 synthesis is upregulated in human nasal polyp tissue. J Allergy Clin Immunol 1997;99:837-42.
11. Simon H-U, Yousefi S, Schranz C, Schapowal A, Bachert C, Blaser K. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J Immunol 1997;158:3902-8.
12. Snedecor W, Cochran G. Statistical methods: the comparison of two samples; analysis of frequencies in one-way and two-way classifications. Ames: The Iowa State University Press; 1982. p.83-106.
13. Sokal R, Rohlf F. Biometry: the principles and practice of statistics in biological research: Estimation and hypothesis testing. San Francisco: W. H. Freeman and Co.; 1969. p.127-174,
14. Billroth R. Uber den Bau der Schleimpolyppen. Vienna: Georg Reimer; 1885. p.1-32.
15. Cauna N, Manzetti GW, Hinderer KH, Swanson EW. Fine structure of nasal polyps. Ann Otol 1972;81:41-58.
16. Dolowitz DA, Dougherty TF. A study of cilia and connective tissue in normal and hyperplastic nasal mucous membrane. Laryngoscope 1966;76:555-59.
17. Drake-Lee A. Nasal polyps in identical twins. J Laryngol Otol 1992;106:1084-5.
18. Jenkins JL. Blockade theory of polyp formation. Laryngoscope 1932;42:703-4.
19. Ohno I, Lea R, Finotto S, Marshall J, Denburg J, Dolovich J, Gauldie J, Jordana M. Granulocyte/Macrophage Colony-stimulating Factor (GM-CSF) Gene Expression by Eosinophils in Nasal Polyposis. Am J Respir Cell Mol Biol 1991;5:505-10.
20. Weille FL. Further experiments in the viral theory of nasal polyp etiology. Ann Allergy 1966;24:549-51.
21. Oppenheimer EH., Rosenstein BJ. Differential pathology of nasal polyps in cystic fibrosis and atopy. Lab Invest 1979;40:445-9.
22. Settipane GA, Chafee FH. Nasal polyps in asthma and rhinitis.. J Allergy Clin Immunol 1977;59:17-21.
23. Jamal A, Marant AGD. Atopy and nasal polyposis. J Laryngol Otol 1987;101:355-8.
24. Moloney JR. Nasal polyps, nasal polypectomy, asthma, and aspirin sensitivity. J Laryngol Otol 1977;81:837-46.
25. Settipane GA, Klein DE, Settipane RJ. Nasal polyps. State of the art. Rhinology 1991:11:33-6.
26. Baumgarten C, Kunkel G, Rudolph R, Stand R D, Sperner I, Gelderblom H. Histopathological examinations of nasal polyps of different etiology. Arch Otorhinolaryngol 1980;226:187-97.
27. Berdal P. Serological examination of nasal polyp fluid. Acta Otolaryngol 1954 (Supplement.) 115:1.
28. Kern RA, Schneck HP. Allergy a constant factor in the etiology of so-called mucous nasal polyps. J Allergy 1933;4:485-97.
29. Samter M, Becker EL. Ragweed reagins in nasal secretions. Proc Soc Exp Biol Med 1947;65:140.
30. Miller CH, Pudiak DR, Hatem F, Looney RJ. Accumulation of interferon gamma-producing TH1 helper T cells in nasal polyps. Otolaryngol Head Neck Surg 1994;111:51-8.
31. Terada N, Konno A, Fukuda S, Yamashita T, Abe T, Shimada H, Yoshimura K, Shirotori K, Ishikawa K, Togawa K. Interleukin-5 Upregulates Intercellular Adhesion Molecule-I Gene Expression in the Nasal Mucosa in Nasal Allergy but not in Nonallergic Rhinitis. Int Arch Allergy Immunol 1995;106:139-45.
32. Castro FFM. Rinite alérgica: modernas abordagens para uma clássica questão. São Paulo: Lemo Editorial; 1997. p.178.
33. Roitt I, Brostoff J, Male D. Immunology. 4th ed. St. Louis: Mosby; 1996. p.89.
34. Haraldsen G, Kuale D, Lien B,Farstad IN, Brandtzaeg P. Cytokine-regulated expression of E-selectin, Intercellular Adhesion Molecule-I (ICAM-I), and Vascular Cell Adhesion Molecule-I (VCAM-I) in human intestinal microvascular endothelial cells. J Immunol 1996;156:2558-65.
35. Petzelbauer P, Bender JR,Wilson J, Pober JS. Heterogeneity of dermal microvascular endothelial cell antigen expression and cytokine responsiveness in sito and in cell culture. J Immunol 1993;151:5062-72.
36. Jahnsen FL, Haraldsen G, Aanesen JP, Haye R, Brandtzaeg P. Eosinophil infiltration is related to increased expression of vascular cell adhesion molecule-I in nasal polyps. Am J Respir Cell Mol Biol 1995;12:624-32.
37. Symon F, Walsh GM, Watson SR, Wardlaw AJ. Eosinophil adhesion to nasal polyp endothelium is P-selectin-dependent. J Exp Med 1994;180:371-6.
38. Beck LA, Stellato C, Beall LD, Schall TJ, Leopold D, Bickel CA, Baroody F, Bochner BS, Schleimer RP. Detection of the chemokine RANTES and endothelial adhesion molecules in nasal polyps. J Allergy Clin Immunol 1996;98:766-80.
39. Bochner BS, Luscinskas FW Jr., Gimbrone MA, Newman W Sterbinsky SA, Derse-Anthony CP, Klunk D, Schleimer RP. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med 1991;173:1553-6.
40. Hamilos DL, Leung DYM, Wood R, Bean DK, Song YL, Schotman E, Hamid Q. Eosinophil infiltration in nonallergic chronic hyperplastic sinusitis with nasal polyposis (CHS/NP) is associated with endothelial VCAM-I upregulation and expression of TNF-a. Am J Respir Cell Mol Biol 1996;15:443-50.
41. Cordell JL, Falini B, Erber WN, Ghosh AK, Abdulaziz Z, Macdonald S, Pulford KAF, Stein H, Mason DY. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem 1984;32:219-29.
42. Min Y, Lee CH, Rhee C, Kim KH, Kim CS, Koh Y, Min K, Anderson PL. Inflammatory cytokine expression on nasal polyps in allergic and infectious rhinitis. Acta Otolaryngol 1997;117:302-6.
1 Ph.D., Professor, Discipline of Otorhinolaryngology, Medical School, University of São Paulo - USP.
2 Post-graduation under course, Department of Otorhinolaryngology, FMUSP.
3 Associate Professor, Discipline of Otorhinolaryngology, FMUSP.
4 Faculty Professor, Discipline of Otorhinolaryngology, FMUSP.
Study conducted at the Discipline of Otorhinolaryngology, FMUSP.
Address correspondence to: Dr. Richard Louis Voegels – Rua Iubatinga, 145 ap. 61-B
São Paulo SP 05716-110 Brazil
Fax (55 11)3749-0719 – E-mail: voegels@attglobal.net
Article submitted on May 13, 2002. Article accepted on July 18, 2002