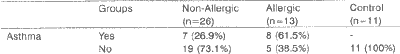Year: 2001 Vol. 67 Ed. 2 - (12º)
Artigos Originais
Pages: 220 to 227
Nasal Polyposis and Allergy: Is There a Correlation?
Author(s):
Richard L. Voegels*,
Patrícia Santoro**,
Ossamu Butugan***,
Lázaro G. Formigoni****,
Aroldo Miniti*****.
Keywords: polyposis, allergy, interleukins 1 , 3, 4, immunoglobulin
Abstract:
Introduction: Nasal polyposis (NP) is a chronic inflammatory disease of the nasal mucosa. The etiology and formation of NP are still not elucidated and Nave been debated for many years. Aim: The objective of the present study was to investigate the role of nasal allergy in the development of NP. The following aspects were analyzed: age, sex and patient's symptoms; correlation between asthma, aspirin intolerance and NP; serum immunoglobulin levels and eosinophilia, and concentration of interleukins 1 , 3, and 4 in NP. Study design: Clinical prospective. Material and method: Thirty nine patients with NP were selected, 13 of them allergic and 26 non- allergic. A control group of 11 individuals was also studied. The concentrations of interleukins 1 , 3, and 4 were measured by enzyme-linked immunosorbent assay (ELISA). Results: There was a higher incidence of NP after the fourth decade of life and among men. We found no correlation of asthma or aspirin intolerance with the presence or absence of allergy. Serum levels of IgE and eosinophils were significantly higher in patients with allergy and NP and the concentrations of interleukins 3, and 4 were positively correlated with NP. There was no difference in interleukin 3 and 4 concentration between the non-allergic group with NP and the control group, suggesting that these interleukins do not play. an important role in the etiology and formation of NP. Conclusion: These results suggest that the immunologic pathway involved in the etiology of NP is different from the one correlated with allergy (IgE mediated).
![]()
INTRODUCTION
Nasal polyposis (NP) is a chronic inflammatory disease of the nasal mucosa that affects about 0.5% of the population9, 43. Pathophysiology of NP is an issue of controversy and there are innumerous theories described in the literature about this affection.
At the beginning of the 20th century, Bourgeois7 published an allergic theory in which the NP would be an inflammatory process derived from some kind of allergy. Since then, a number of other theories have arisen in order to try to elucidate the mechanisms involved in the genesis of NP5,12,33,49. However, different authors continue to define NP as an allergic process, especially because of the intense eosinophllia present in the polyp tissue8,15,19.
In recent years, thanks to the significant advance of immunology, a number of authors have tried to identify immune mediators involved in the genesis of NP in order to try to define its possible origin.
In order to understand development and immune mechanisms of NP we decided to conduct a study with two groups of patients with NP: a group of allergic patients and a group of non-allergic patients, plus a control group for comparison. The purpose of the present study was to investigate:
o the correlation between asthma, intolerance to acetyl salicylate acid and nasal polyposis;
o serum levels of immunoglobulins (IgA, IgE, IgG and IgM) and eosinophils;
o concentration of interleukins 1 , 3 and 4 in nasal polyps.
MATERIAL AND METHOD
We studied prospectively 39 patients with nasal polyposis, treated and operated in the second semester of 1997 in the Division of Clinical Otorhinolaryngology at Hospital das Clínicas, Faculdade de Medicina da Universidade de São Paulo. In the group, 22 patients were male (56.4%) and 17 (43.6%) were female patients. Ages ranged form 42.6 ± 16.9 years (16 years to 88 years) (mean ± standard deviation).
We used the following criteria for inclusion and exclusion of patients:
o Inclusion criteria: We included only patients who had diagnosis of bilateral nasal polyposis that would be submitted to surgical treatment.
o Exclusion criteria: We excluded patients who had one single polyp (antrochoanal, sphenochoanal) or other diseases related to nasal polyposis, such as cystic fibrosis, primary ciliary diskinesia and fungal sinusitis. Lastly, patients who could not be classified as allergic or non-allergic were also dismissed.
The 39 patients selected as shown above were divided into two groups:
allergic group (13 patients):
• typical history of allergic rhinitis (nasal pruritis, sneezing, rhinorrhea, nasal obstruction);
• positive cutaneous test of hypersensitivity.
non-allergic group (26 patients):
• absence of allergic rhinitis; .
• negative cutaneous test of hypersensitivity.
control group: It was formed by 11 volunteer adults who had no previous history of nasal and/or allergic symptomatology. The nasal fossa exam with rigid endoscope showed normal results. In this group, 7 subjects (63.6%) were male and 4 (36.4%) were female subjects. Ages ranged from 29.8 ± 5.3 years (25-42 years) (mean ± standard deviation).
All patients were evaluated one week before surgery and patients were asked to score from 1 to 10 the following symptoms: headache, nasal obstruction, hyposmia, purulent secretion, nasal discharge and cough. We considered score 1 an asymptomatic status and score 10 as severe symptoms. We also collected blood for dosage of IgA, IgG, IgM and IgE and eosinophil, in addition to allergic cutaneous test.
The collection of fragments of nasal polyps was conducted during the surgery under general anesthesia. We collected two fragments from the polyps of each patient and one was submitted to hematoxylin and eosin staining and the second was cut in small pieces and centrifuged for 10 minutes at 3,000 rotations per minute. The supernatant was separated and kept in the freezer at -70° C until the conduction of the test with ELISA immunoenzymatic assay2,44.
The collection of fragments from the normal nasal mucosa of the control group was conducted with Grünwald forceps. We removed mucosa from the middle concha under rigid endoscope view of 4.0mm at 30°. The mucosa of these subjects was submitted to the same methods described for the polyp mucosa.
The determination of interleukin-1 concentration (IL-1 ), 3 and 4 in nasal polyp macerate was conducted by immunoenzymatic essay using a commercially available kit (R&D - Minneapolis, USA -Lot 9716605). In the control groups, interleukins 1, 3 and 4 were dosed in a macerate of normal nasal mucosa.
Upon analyzing the results, we studied and correlated the following data:
• age and gender of patients;
• asthma and intolerance to acetyl salicylate acid;
• clinical picture;
• eosinophils and serum immunoglobulins (total IgE, IgA, IgG and IgM);
• ELISA (interleukins 1 , 3 and 4).
The comparison of groups was conducted using parametric and non-parametric statistical tests, taking into consideration the nature of data and variability of measures made. We applied all statistical studies with the predetermined significance level of 5%45,46.TABLE 1 - Distribution of mean, standard deviation and amplitude of age of patients in the studied groups.
Key: SD - standard deviation. There was no statistically significant difference (pE 0.05) between allergic and non-allergic groups. Statistical test used: student t test.
TABLE 2 - Distribution of gender of patients in the studied groups.
Key: There was no statistically significant difference (p6 0.05) among the three groups. Statistical test used: qui-square test.
TABLE 3 - Distribution of incidence of asthma in the studied groups.
Key: SD - standard deviation. Marked values are statistically significant (p< 0.05). Statistical test used: qui-square test.
RESULTS
The age range of patients varied from 16 to 88 years (Table 1).
As to gender, considering all studied patients, there was a slight predominance of NP in male gender (Table 2).
We observed a statistically significant higher incidence (p< 0.001) of asthma among allergic patients (Table 3). Of the 15 patients with asthma, 11 (73.3%) were over 30 years of age, and the mean onset age of asthma was 34.6 years.
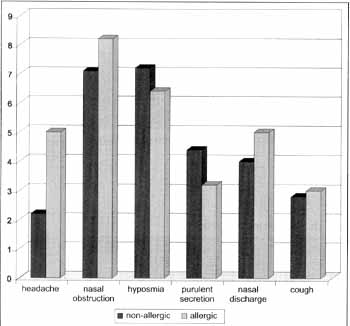
Based on clinical history, we detected intolerance to aspirin in four patients, two in each group (allergic and non-allergic). Therefore, there was no statistically significant difference in the incidence of aspirin intolerance between the groups. Upon comparing the mathematical means of scores of clinical picture of allergic and non-allergic patients, we did not observed statistically significant difference between the groups in any of the symptoms (Graph 1).
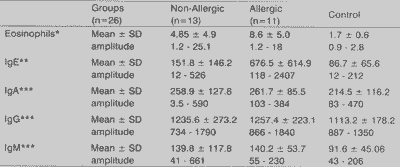
Serum levels of eosinophils and immunoglobulins are represented in Table 4. There was no statistically significant difference among the 3 groups for IgA, IgG and IgM. However, for eosinophils and IgE, the group of allergic patients presented statistically significant higher levels (p < 0.001) than the other two groups.
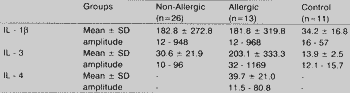
Concentrations of IL-1 found in the nasal secretion were higher in patients with NP (allergic and non-allergic) when compared to the control group. However, it was not a statistically significant difference (Table 5).
As to interleukins 3 and 4, the results were similar. We observed that the allergic group presented levels significantly higher of these interleukins, whereas the other two groups (control and non-allergic) did not present relevant differences.
Graph 1. Evaluation of clinical picture by patients. Note: There was no statistically significant difference (p < 0.05) between Allergic and Non-Allergic groups. Statistical test used: Mann Whitney test.
Histologic exam using hematoxylin eosin staining showed intense eosinophilic infiltrate in 37 (94.8%) of the 39 patients with NE In two of them, we did not observe relevant tissue eosinophilia and both of them belonged to the non-allergic group.
DISCUSSION
The exact pathophysiology of NP remains unknown5,6,11,12,13,14,22,34,49,50. The patients who present it have a complex and intense inflammatory infiltrate with presence of innumerous chemical mediators19,35.
Among patients with NP, there was a predominance of subjects over 30 years of age, represented by almost 75% (29/39) of the total. Such data confirm the data reported in the literature. Settipane and Chafee4l, in a review of 149 cases, found 122 cases (82%) over the age of 30 years. Similar information was reported by Jamal; Marant20 and Granstrom et al.15 suggested high prevalence of this disease after the third decade of life.
We did not find statistically significant difference in age of patients in the two studied groups - allergic and nonallergic. The mean age in the allergic group was 37.3 years and in the non-allergic group it was 45.3 years.
It is interesting to notice that, similarly to the literature, we found few cases in the first two decades of life, suggesting that except for patients with cystic fibrosis, NP rarely affects patients in this age range.TABLE 4 - Serum concentration of eosinophils and immunoglobulins E, A, G and M found in the studied groups.
Key: SD - standard deviation.
* values in percentage (%) of total of white cells.
** values expressed in international units/milliliter (IU/mL).
*** values expressed in milligram/ deciliter (mg/dL).
Values marked if statistically significant (p5 0.05).
Statistical tests used: Kruskal Wallis test (eosinophils and IgE).
Variance analysis (IgA, IgG and IgM).
TABLE 5 - Concentration of cytokines IL-1 , 3 and 4 found in studied groups (values in picogram/milliliter).
Key: SD - standard deviation. Values marked if statistically significant (p < 0.05)
Statistical tests used: Kruskal Wallis test (IL-1 ). Variance analysis (IL-3).
Out of a total of 39 patients with NP, 22 (56.4%) were male. These data confirmed the information provided by the literature, showing predominance of male gender15,20,29.
Patients with NP frequently present associated chronic sinusitis and their clinical picture is normally characterized by headache, nasal obstruction, hyposmia, purulent secretion, nasal discharge and cough.
Although some authors suggest that the presence of allergy associated with NP may exacerbate the symptomatology of these patients42, we did not find statistically significant difference between the groups concerning clinical presentation. In both, the main symptoms were nasal obstruction and hyposmia. The groups of allergic patients presented worse scores in headache, nasal obstruction, nasal discharge and cough. However, there was no statistically significant variation for any of the symptoms.
The role of atopy in the pathophysiology of NP has been a topic of controversy for many years. Different authors believe that allergy has a key role in the formation of polyps3,4,8,15,19,23,39, However, recent studies with cytokines suggested the opposite 27,44,48. Taking the issue into consideration, we divided our patients in two groups - allergic and non-allergic. Allergic subjects amounted to 33.3% of the total of patients with NP, which corresponds to the incidence of atopy in the general population of a large urban center, such as São Paulo10.
Settipane et al. 42 reinforced that although NP is not more frequent in atopic patients, if both pathologies coexist, there is an exacerbation of NP.
The association of asthma and NP has been recognized for many ears29, 38, 41.
The incidence of NP in the general population seems to be of 0.2% to 1.0%43. Studies in cadavers demonstrated an incidence of NP of up to 42%24. On the other hand, the incidence of asthma in the population as a whole is about 0.5%43.
In our sample, we found 38.4% (15/39) of asthmatic patients, in accordance with the data provided by the literature. When we compared the groups of patients, the incidence of asthma was much higher in the group of allergic patients 61.5 % (8/13) against 24.1% (7/29) in the non-allergic group. This difference was significant (p>0.001) between the groups; thus, asthma is much more common in allergic patients. This information suggested that the presence of allergy in NP patients may facilitate development of asthma.
In a number of studies, it has been emphasized that the onset of asthma takes place mainly after the third decade of life24,29,36, mean manifestation at 35 years. In our study, there was also a higher incidence of the disease as from the 3rd decade of life. Out of 15 patients with asthma, 11 (73.3 %) were over 30 years and the mean onset age was 34.6 years.
The occurrence of aspirin intolerance with NP varies from 3 % to 26%, depending on the study15,21,24,19. Among our patients, 11.1% (4/36) manifested intolerance to aspirin, which is compatible with the figures found in the literature. Curiously, all of them also had concomitant asthma presentation, forming the classical triad described by Widal et al.51.
In the literature, we did not find a study correlating concentrations of immunoglobulins A, G, M (serum and/ or tissue) and presence or not of NP In our patients, results were similar in each group, and there was no significant difference among them. The data suggested that immunoglobulins do not seem to have an expressive role in the pathophysiology of NP.
Interleukin 1 was denominated endogenous pyrogen, activator factor of lymphocytes and cataboline. It is produced by various cells, such as endothelial, lymphocyte B, fibroblast and macrophage cells. One of its main functions is to stimulate lymphocytes B and T to induce inflammatory reaction. Other functions of IL-1 includes induction to fever by stimulating the CNS and stimulation of release of corticoid in adrenal glands. Practically all cells of the body respond to IL-1 stimuli, by means of specific receptors on the membrane cell. Therefore, IL-1 is a nonspecific cytokine present in large amounts whenever there is a significant inflammatory process37.
In our study, we decided to dose the fraction- of IL1 that corresponded to the active fraction of the cytokine. The presence of IL-1 in the tissue of nasal polyp was demonstrated by Hamaguchi et al.16 and Mullol et al.30. However, these authors did not present control group in order to compare results, which did not allow confirmation of whether the levels of IL-3 found were significant or not.
During our study, we obtained much higher levels of IL-1 for patients with NP (allergic or non-allergic) when compared to the control group, suggesting that the presence of IL-1 is related with chronic inflammatory process present in the tissue of NP, and there was no relation with allergy. However, the difference among the control group and the other two groups did not reach statistically significant levels and we could not draw final conclusions about this diversity.
Interleukin 3, also known was multi-specific hemopoietin, stimulates the growth of precursors of all hemopoietic lines and it is mainly produced by the lymphocyte T37.
IL-3 plays an important role in the migration and activation of eosinophils in allergic processes26. Its specific action in NP, especially over the eosinophils present in the pathology, is still a controversial issue.
Hamilos et al.18 demonstrated, using in situ hybridization of mRNA, that in NP tissue there was intense eosinophilia and that eosinophils present were positive to mRNA IL-3. Allen et al.1, using immunohistochemistry found similar results. The authors suggested that in this tissue, IL3 was produced by the eosinophils.
On the other hand, Bachert et al.² analyzed polyps in 23 patients using ELISA and did not observe significantly higher levels of IL-3 in the tissues.
In our sample, we found significantly higher levels of IL-3 only in the group with allergic patients (p>0.001). The group of non-allergic patients showed high values of IL-3 when compared to the control group, but the difference was not statistically significant, in accordance with the results by Bachert et al.2. Such results suggested that IL-3 may have an important role in the pathophysiology of allergy, but it does not seem to be involved in the pathophysiology of NE
The authors who found increase of expression of IL3 in patients with NP did not define if the patients had allergy or not1,18. Therefore, it is possible that the high levels of IL-3 observed by the authors were a result of the presence of allergy among their patients.
Interleukin 4 is known as lymphocyte B activator factor. Its main characteristic is induction and activation of lymphocytes B for the production of IgE37. Another important function of IL-4 is to transform lymphocyte T helper (Th) into lymphocyte T helper 2 (Th2). The increase in production of Il-4 is a characteristic of allergic reactions3l,40, causing elevation of IgE concentration47.
There is no consensus as to the presence and role of IL-4 in NE On the one hand, studies such as the ones by Nonaka et al.32 and Min et al.28 suggested that IL-4 would be increased in nasal polyp tissues; on the other hand, different authors17,27 did not detect increase of IL-4 in tissue of nasal polyp in non-allergic patients. It is important to point out that in the Nonaka et al.32 study there was no differentiation among allergic and non-allergic patients, and maybe IL-4 was increase because there were allergic patients in the group. In the research by Min et al.28, despite the data of elevated 11,4 in the NP tissue of non-allergic patients, the authors suggested that the mechanism that would lead to the growth would be different from that typical of type I hypersensitivity.
In our sample, there was no statistically significant variation in values of IL-4 found in normal tissue of the nasal mucosa of the control group nor in the nasal polyp tissue of the non-allergic patients, because dosages conducted for both groups were negative. On the other hand, when we compared the values of IL-4 found in the nasal polyp tissue of allergic patients with those of non-allergic patients, we observed a statistically significant difference (p<0.001); there was only one dosage of IL-4 in a allergic patients that was negative.
There is still a strong correlation among these data and serum level of IgE. Despite the absence of relevant difference in the serum level of IgE between the control group and the non-allergic patients, the serum level of IgE found in allergic patients was statistically significantly higher when compared to the control group (p < 0.001) and the non-allergic patients (p<0.001). It is interesting to observe that the only allergic patient that had normal levels of IgE was the one that had negative dosage of IL-4.
Our results suggested that there is a strong correlation of IL-4 with the presence of allergy and high serum levels of IgE; moreover, the role of IL-4 in eosinophilia found in NP is probably not important.
Therefore, by analyzing the studied factors, we could observed that there was no correlation between asthma or intolerance to aspirin and presence or not of allergy. Serum levels of IgE and eosinophils were significantly higher in allergic patients with nasal polyposis and presented a direct correlation with concentrations of interleukins 3 and 4 in the nasal polyp. There was no difference between the group of non-allergic with nasal polyposis and the control group concerning levels of interleukins 3 and 4, suggesting that these interleukins do not have an important role in the pathophysiology of nasal polyposis. The data suggested that NP has a immunological mechanism different from that of allergic processes.
CONCLUSION
1) Presence or not of allergy in patients with nasal polyposis was not related to presence of asthma or intolerance to aspirin.
2) Serum levels of IgA, IgG and IgM were the same in all groups, regardless of the presence of nasal polyposis.
3) Serum levels of IgE and eosinophils were related with the presence of nasal polyposis and allergy.
4) Concentrations of interleukins 3 and 4 had direct relation with the presence of nasal polyposis and allergy.
REFERENCES
1. ALLEN, J.S.; EISMA, R.; LEONARD, G.; KREUTZER, D. Interleukin-3, Interleukin-5, and granulocyte-macrophage colony-stimulating factor expression in nasal polyps Am. J. Otolaryngol, 18, 239-46, 1997.
2. BACHERT, K.; WAGENMANN, M.; HAUSER, U.; RUDACK, C. IL-5 synthesis is upregulated in human nasal polyp tissue. J Allergy Clin. Immunol, 99, 837-42, 1997.
3. BAUMGARTEN, C.; KUNKEL, G.; RUDOLPH, R.; STAND, R. D.; SPERNER, I.; GELDERBLOM, H. Histopathological examinations of nasal polyps of different etiology. Arch. Otorhinolaryngol, 226, 187-97, 1980.
4. BERDAL, E Serological examination of nasal polyp fluid. Acta Otolaryngol. (Supplement.), 115, 1, 1954.
5. BERNSTEIN, J. M.; GORFIEN, J.; NOBLE, B.; YANKASKAS, J. R. Nasal polyposis: Immunohistochemistry and bioelectrical findings (a hypothesis for the development of nasal polyps). J Allergy Clin. Immunol., 99, 165-75, 1997.
6. BILLROTH, R. Uber den Bau der Schleimpolyppen. Vienna, Georg Reimer, 1885, 1-32.
7. BOURGEOIS, H. A propos d"un cas de coryza spasmodique. Progr. Med., 95-6, 1925.
8. CALENOFF, E.; GUILFORD, T.; GREEN, J.; ENGELHARD, C. S. Bacteria-specific IgE in patients with nasal polyposis. Arch. Otolaryngol, 109, 372-5, 1983.
9. CAPLIN, I.; HAYNES, J. T.; SPAHN, J. Are nasal polyps an allergic phenomenon? Ann. Allergy, 29, 631-4, 1971.
10. CASTRO, F.F.M. Rinite alérgica: modernas abordagens para uma clássica questão. São Paulo, Lemo Editorial, 1997, 178.
11. CAUNA, N.; MANZETTI, G. W; HINDERER, K. H.; SWANSON, E. W Fine structure of nasal polyps. Ann. Otol., 81, 41-58, 1972.
12. DAVISON, F. W Hyperplastic sinusitis, a five year study. Ann. Otol, 72, 462-74, 1963.
13. DOLOWITZ, D. A.; DOUGHERTY, T F. A study of cilia and connective tissue in normal and hyperplastic nasal mucous membrane. Laryngoscope, 76, 555-59, 1966.
14. DRAKE-LEE, A. Nasal polyps in identical twins. J. Laryngol. Otol, 106, 1084-5, 1992.
15. GRANSTRÖM, G.; JACOBSSON, E. JEPPSSON, P H. Influence of allergy, asthma and hypertension on nasal polyposis. Acta Otolaryngol., 492, 22-7, 1992.
16. HAMAGUCHI, Y; SUZUMURA, H.; ARIMA, S.; SAKAKURA, Y Quantitation and immunocytological identification of interleukin-1 in nasal polyps from patients with chronic sinusitis. Int. Arch. Allergy Immunol., 104, 155-9, 1994.
17. HAMILOS, D.L.; LEUNG, D.YM.; WOOD, R.; CUNNINGHAM, L.; BEAN, D.K.; YASRUEL, Z.; SCHOTMAN, E.; HAMID, Q. Evidence for distinct cytokine expression in allergic versus nonallergic chronic sinusitis. J. Allergy Clin. Immunol, 96, 537-44, 1995.
18. HAMILOS, D.L.; LEUNG, D.YM.; WOOD, R; MEYERS, A.; STEPHENS, JX; BARKANS, J.; MENG, Q.; CUNNINGHAM, L.; BEAN, D.K.; KAY A.B.; HAMID, Q. Chronic hyperplastic sinusitis: association of tissue eosinophilia with mRNA expression of granulocyte-macrophage colony-stimulating factor and interleukin-3. J Allergy Clin. Immunol, 92, 39-48, 1993.
19. JACOBS, R. L.; FREDA, E. J.; CULVER, W G. Primary nasal polyposis. Ann. Allergy, 51, 500-5, 1983.
20. JAMAL, A.; MARANT, A. G. D. Atopy and nasal polyposis. J. Laryngol Otol, 101, 355-8, 1987.
21. JANTTI-ALANKO, S.; HOLOPAINEN, E.; MALMBERG, H. Recurrence of nasal polyps after surgical treatment. Rhinology (Supplement.), 8, 59-64, 1989.
22. JENKINS, J. L. Blockade theory of polyp formation. Laryngoscope, 42, 703-4, 1932.
23. KERN, R. A.; SCHNECK, H. E Allergy a constant factor in the etiology of so-called mucous nasal polyps. J. Allergy, 485-97, 1933.
24. LARSEN, K.; TOS, M. Clinical course of patients with primary nasal polyps. Acta Otolaryngol (Stockh) Supplement., 114, 1994, 556-9.
25. LARSEN, EL.; TOS, M. Anatomic side of origin of nasal polyps: endoscopic nasal sinus surgery as a screening method for nasal polyps in an autopsy material. Am. J. Rhinol., 10, 211-6, 1996.
26. LIU, C.M.; SHUN, C.T; CHENG, YK. Soluble adhesion molecules and cytokines in perennial allergic rhinitis. Ann. Allergy Asthma Immunol., 81, 176-80, 1998.
27. MILLER, C.H.; PUDIAK, D.R.; HATEM, F.; LOONEY, R.J. Accumulation of interferon gamma-producing TH1 helper T cells in nasal polyps. Otolaryngol. Head Neck Surg., 111, 51-8, 1994.
28. MIN, Y; LEE, C.H.; RHEE, C.; HIM, K.H.; HIM, C.S.; KOH, Y; MIN, K.; ANDERSON, PL. Inflammatory cytokine expression on nasal polyps in allergic and infectious rhinitis. Acta Otolaryngol., 117, 302-6, 1997.
29. MOLONEY, J. R. Nasal polyps, nasal polypectomy, asthma, and aspirin sensitivity. J. Laryngol. Otol., 81, 83746, 1977.
30. MULLOL, J.; XAUBET, A.; GAYA, A.; ROCA-FERRER, J.; L6PEZ, E.; FERNANDEZ, J.C.; FERNANDEZ, M.D.; PICADO, C. Cytokine gene expression and release from epithelial cells. A comparison study between healthy nasal mucosa and nasal polyps. Clinical Exp. Allergy, 5, 1994, 607-15.
31. NACLERIO, R.M. Pathophysiology of perennial allergic rhinitis. Allergy (Supplement.), 52, 7-13, 1997.
32. NONAKA, M.; NONAKA, R.; WOOLLEY, K.; ADELROTH, E.; MIURA, K.; OKHAWARA, Y; GLIBETIC, M.; NAKANO, K.; O'BYRNE, P; DOLOVICH, J.; JORDANA, M. Distinct immunohistochemical localization of IL-4 in human inflamed airway tissues. J. Immunol., 155, 3234-44, 1995.
33. NORLANDER, T.; FUKAMI, M.; WESTRIN, K. M.; STIERNA, E; CARLSOO, B. Formation of mucosal polyps in the nasal and maxillary sinus cavities by infection. Head Neck Surg., 109, 522-9, 1995.
34. OHNO, I.; LEA, R.; FINOTTO, S.; MARSHALL, J.; DENBURG, J.; DOLOVICH, J.; GAULDIE, J.; JORDANA, M. Granulocyte/Macrophage Colony-stimulating Factor (GMCSF) Gene Expression by Eosinophils in Nasal Polyposis. Am. J. Respir Cell Mol. Biol., 1991, 505-10.
35. OPPENHEIMER, E. H.; ROSENSTEIN, B. J. Differential pathology of nasal polyps in cystic fibrosis and atopy. Lab. Invest., 1979, 445-9.
36. PEARSON, B. R. S. Hypersensitivity to aspirin. In: DIXON, A.; MARTIN, K. B., ed. Salicylates. London, J&A. Churchill, 1963, 170.
37. ROITT, L; BROSTOFF, J.; MALE, D. Immunology. 4.ed. Mosby, (St. Louis), 1996, 89.
38. SAMTER, M.; BEERS, R. F. Concerning the nature of intolerance to aspirin. J. Allergy, 40, 281-93, 1967.
39. SAMTER, M.; BECKER, E.L. Ragweed reagins in nasal secretions. Proc. Soc. Exp. Biol. Med., 65, 140, 1947.
40. SCHLEIMER, R.R; STERBINSKY S.A.; KAISER, J.; BICKEL, C.; KLUNK, D.; TOMIOKA, K.; NEWMAN, W; LUSCINSKAS, F.W; GIMBRONE, MAJR.; MCINTYRE, B.W; BOCHNER, B.S. IL-4 induces adherence of human eosinophils and basophils but not neutrophils to endothelium. J. Immunol., 148, 108692, 1992.
41. SETTIPANE, G. A.; CHAFEE, F. H. Nasal polyps in asthma and rhinitis. J. Allergy Clin. Immunol. , 59, 17-21, 1977.
42. SETTIPANE, G. A.; KLEIN, D. E.; SETTIPANE, R. J. Nasal polyps. State of the art. Rhinology, 11, 33-6, 1991.
43. SETTIPANE, GA.; LUND, VJ.; BERSTEIN, J.M.; TOS, M. Nasal polyps: epidemiology, pathogenesis and treatment. Rhode Island, Oceanside Publications, 1997, 97.
44. SIMON, H-U.; YOUSEFI, S.; SCHRANZ, C.; SCHAPOWAL, A.; BACHERT, C.; BLASER, K. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J. Immunol., 158, 3902-8, 1997.
45. SNEDECOR, W; COCHRAN, G. Statistical methods: the comparison of two samples; analysis of frequencies in one way and two-way classifications. Ames, The Iowa State University Press, 1982, 83-106.
46. SOKAL, R.; ROHLF, F. Biometry: the principles and practice of statistics in biological research: Estimation and hypothesis testing. San Francisco, W H. Freeman and Co., 1969, 127-174.
47. TEPPER, R.I.; LEVINSON, D.A; STANGER, B.Z.; CAMPOS-TORRES, J.; ABBAS, A.K.; LEDER, E IL-4 Induces Allergic-like Inflammatory Disease and Alters T Cell Development in Transgenic Mice. Cell, 62, 457-67, 1990.
48. TERADA, N.; KONNO, A.; FUKUDA, S.; YAMASHITA, T; ABE, T.; SHIMADA, H.; YOSHIMURA, K.; SHIROTORI, K.; ISHIKAWA, K.; TOGAWA, K. Interleukin-5 Upregulates Intercellular Adhesion Molecule-I Gene Expression in the Nasal Mucosa in Nasal Allergy but not in Nonallergic Rhinitis Int. Arch. Allergy Immunol., 106, 139-45, 1995.
49. TOS, M. The pathogenetic theories in formation of nasal polyps. Am. J. Rbinol., 4, 51-56, 1990.
50. WEILLE, F. L. Further experiments in the viral theory of nasal polyp etiology. Ann. Allergy, 24, 549-51, 1966.
51. WIDAL, M.F.; ABRAMI, E; LERMOYEZ, J. Anaphylaxie et idiosyndraise. Press Med., 30, 189, 1922.
* Director of Rhinology of the Department of Otorhinolaryngology at HCFMUSP and Ph.D. of the Discipline of Otorhinolaryngologyat FMUSP
** Postgraduate Physician of the Department of Otorhinolaryngology at FMUSP
*** Associated Professor of the Discipline of Otorhinolaryngology at FMUSE
**** Professor of the Discipline of Otorhinolaryngology at FMUSE
***** Faculty Professor of the Discipline of Otorhinolaryngology at FMUSE
Study conducted at the Department of Ophthalmology and Otorhinolaryngology at FMUSP, presented at Congresso Triológico de ORL, held in São Paulo, in November 1999, awarded with "Ermírio de Lima" prize, as the best study in the area of Rhinology.
Address for correspondence: Dr. Richard Louis Voegels - Rua Iubatinga, 145 apto. 61-B - 05716-110 São Paulo /SP - Brazil - Fax: (55 11) 3749-1029. E-mail: voegels@attglobal.net
Article submitted on October 20, 2000. Article accented on December 7. 2000.