Year: 2000 Vol. 66 Ed. 6 - (13º)
Artigos Originais
Pages: 660 to 664
An Open Multicenter Study Using Broncho-Vaxom® as an Adjunt Therapy for the Treatment a Prevention of Respiratory Community Aquired Infections in Children.
Author(s):
Shirley S. N. Pignatari*,
Luc L. M. Weckx**,
Aldo C. Stamm***.
Keywords: Broncho-Vaxom®, imimostimulant
Abstract:
Introduction: Controlled clinical studies in Europe have already shown-the efficacy of Broncho-Vaxom® (an oral immunomodulating Material lysate) for the treatment and prevention of bronchitis, sinusitis, pharyngitis and otitis media in children. However, it is not of our knowledge any studies of Broncho-Vaxom® in children with respiratory infections in our population. Aim: In order to validate, the efficacy and safety of Broncho-Vaxom® the authors evaluated the treatment of 792 children assisted in several medical centers in Brasil, ages 6 months to 12 years, referred for recurrent otitis media, recurrent sinusitis, recurrent bronchitis/pneumonia and recurrent pharyngitis/tonsilitis. Material and methods: The patients were evaluated both by subjetive and objective criteria. The diagnosis was based on a standardized history and physical examination and roentgenograms. Broncho-Vaxom® was prescribed for 10 days (3,5 mg/day once a day), for 3 consecutive months. They were reevaluated at 30, 60 and 90 days after the first day of medication. Results: Overall, most of the patients presented excellent and good results, with absence of infections in 90 days, and/or increasing the period between infections and/or reducing the gravity or the time lasting of infections. Conclusion: Overall, minor adverse reactions were observed in 6% dos patients (vomit, diarrhea, nausea). There was no report of interruption of the treatment.
![]()
INTRODUCTION
Considered very frequent affections in childhood, respiratory infections are the most common cause of administration of antibiotics to this population. Bacterial etiological agents of acute otitis media and rhinosinusitis are well established and the most important microorganisms are Pneumococo, Haemophilus influenzae, and finally Moraxella catarrhalis3, 13.
Pneumonia, although it is a lower airway infection that may affect bronchia, bronchiole, alveoli, pulmonary interstitium, and sometimes, the pleural recovering layer, share the same most common etiological bacterial agents in childhood: Pneumococo and Haemophilus influenzae4.
As to pharyngotonsillitis, the most common bacterial agent is still Streptococcus Beta Hemolytic of Group A, responsible for at least 30% of the cases in our country6.
Although they are clinically distinct, respiratory infections frequently present common triggering factors, especially episodes of cold and viral infections (upper airway infections), and share similar bacterial etiology, sometimes in a concomitant episode. Currently, there is much concern as to the increase of resistant microorganisms, and a large percentage of Haemophilus and Moraxella catarrhalisthat produce B lactamase has been observed in various studies; there is also a small emerging percentage of penicillin-resistant Pneumococo, posing a problem as to the appropriate selection of antimicrobial therapy2, 3, 11. It is believed that the excessive empirical use of antimicrobial agents for the treatment of these affections is a factor that contributes to these changes of the bacterial flora11.
Bacterial extracts have been proposed for years now in order to stimulate the response of the immune system, enhancing the protection against infections. Broncho-Vaxo® is a bacterial lysate consisting of fractions of the following bacteria: Haemophilus influenzae, Streptococcus pneumoniae, Klebsiella pneumoniae a ozaenae, Staphylococcus aureus, Streptococcuspyogenesa viridans, Moraxella catarrhalis, and it acts stimulating the natural defenses of the organism. Many studies have shown that Broncho-Vaxom® reduces the frequency and duration of respiratory infections in children, lessen the severity of symptoms and enables reduction of time and use of antibiotic therapy®. Scientific researches have also shown that Broncho-Vaxom® acts as a significant factors in the improvement of immunological parameters, involving non-specific immune system. It has been demonstrated that these extracts lead to stimulation of NADPH, expression of adhesion molecules, as well as to synthesis of cytokines, such as TNF-alpha and IL-2, in addition to stimulating production of IgA in the pulmonary secretions5, 6, 9, 12, 15.
In the present study, the authors carried out an assessment of efficacy and tolerance of Broncho-Vaxom® in the adjuvant treatment and prevention of recurrent respiratory infections in children.
MATERIAL AND METHOD
We evaluated 792 children, aged between 6 months and 12 years, with diagnosis of recurrent otitis media (49 patients), recurrent sinusitis (62 patients), bronchitis/recurrent pneumonia (42 patients) and recurrent pharyngotonsillitis (159 patients). Four hundred and eighty children had associated recurrent infections.
We included in the study children who had consistent clinical history of respiratory tract recurrent infections - at least 3 episodes in the previous 6 months or 4 or more episodes in the previous year. We excluded patients younger than 6 months, those who had immune debilitating diseases, craniofacial malformations, or who had used immunostimulating therapy in the previous 6 months. Diagnosis of otitis media was based on clinical picture, and we considered as minimum criteria the presence of otalgia followed by two or more signs and symptoms: irritability/fever, otorrhea and characteristic otoscopy, reduction of mobility of tympanic membrane, hyperemia/ opacification. Diagnosis of sinusitis was based on the clinical picture and included: nasal obstruction followed by 2 or more parameters - mucous-purulent rhinorrhea, fever and cough, followed by peri-orbital edema and/or headache and compatible radiological exam; diffuse opacification or liquid level of mucous thickness higher than 4mm, and/or nasal endoscopic exam showing purulent secretion in the middle meatus.
As to diagnosis of pneumonia/bronchitis, we considered cough associated to two or more signs and symptoms: fever, compatible auscultation, irritability, reduction of appetite, mucous-purulent expectoration, and simple chest x-ray compatible with bacterial infection.
For the diagnosis of pharyngotonsillitis, we considered sore throat associated to -at least two more parameters: edema, hyperemia, exsudate, fever, cervical ganglia, irritability and reduction of appetite.
After the person responsible for the patient signed the informed consent, children were medicated with BronchoVaxom® at a dose of 3.5mg/day, in a, 10-day regimen for 3 consecutive months.
Efficacy of treatment was checked by the presence or absence of infections, increase in intervals between episodes and reduction of duration of infections, as well as severity of symptoms. It was checked in visits 30, 60 and 90 days after the beginning of treatment. We considered as attained objective when the conditions described above were reported, and as unattained objective when there was maintenance or worsening of studied parameters. Evaluation of treatment was conducted subjectively as well by the responsible caregiver, and the research investigator, as excellent, good, fair and unsatisfactory.TABLE 1 - Demographic characteristics of studied children.
Compliance to treatment was evaluated based on use or not of medication, controlled by the return of the drug packages.
Tolerance to medication was checked by the spontaneous report about side effects in the 3 visits after the beginning of medical treatment.
RESULTS
We included 792 children in this multicenter study. They had recurrent respiratory infections as follows: 49 had history of otitis media, 62 had sinusitis, 159 had pharyngotonsillitis, 42 had bronchitis/pneumonia; 480 had associated infections.
Demographic characteristics of the studied children
Ages ranged from 6 months to 12 years, mean age of 5.1 years, and 437 patients (55.2%) were boys and 355 (44.8%) were girls (Table 1).
Evaluation of efficacy of treatment
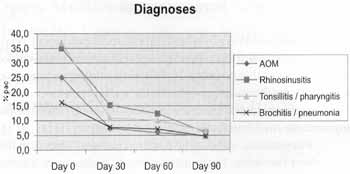
a) Number of infections during the evaluation period Objective evaluation of infectious episodes showed that the percentage of patients who had infections in the evaluation period reduced significantly after the 30th day of the' study (Figure 1).
b) Attained objectives
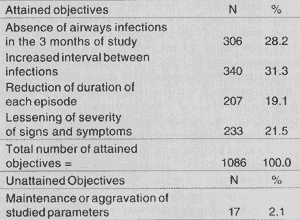
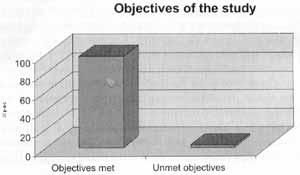
Objectives were attained in 97.9% of the cases (absence of infections for the 3 months of study and/or increased interval between episodes and/or reduction of duration of each episode, and/or lessening of severity of signs and symptoms) (Table 2 and Figure 2).
Compliance to treatment
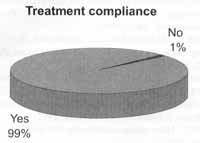
Treatment compliance was obtained in 98.9% of the cages, that is to say, 780 patients used medication as prescribed by the physician (Figure 3).
Figure 1. Diagnosis of infection in intervals and at the assesstment*.
* Friedman's test: significant from day 30.
TABLE 2 - Percentage of children who presented absence of infections, increased interval between them, reduction of duration and lessening of signs and symptoms of infectious episodes.
Figure 2. Percentage of patients that attained the objectives of the study.
Figure 3. Percentage of patients that were compliant with the proposed treatment.
Evaluation of treatment according to the person responsible for the patient
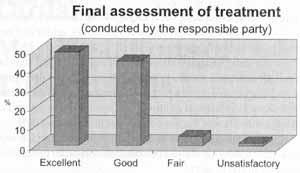
In the subjective evaluation of the person responsible for the patient, the treatment was considered excellent or good in 93.6% of the cases, fair in 4.7% and unsatisfactory in 1.7% (Figure 4).
Evaluation of treatment according to research physician
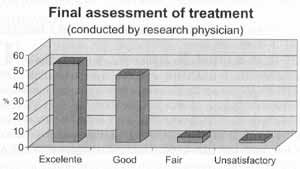
The researcher classified subjectively the treatment with Broncho-Vaxom® as excellent or good in 95.2% of the cases, fair in 3.3% and unsatisfactory in 1.5% (Figure 5).
Tolerance to Broncho-Vaxom®
We observed side effects in 6,3% of the patients. The most frequent complaints were diarrhea, vomiting, epigastralgia and nausea.
DISCUSSION
Community-acquired respiratory infections are frequently diagnosed in the childhood population, a reason for many medical visits and antibiotic prescriptions; they normally present the peak of incidence during winter, generally preceded by an episode of upper airway infection. Although they represent an important health problem in children, correct diagnosis and optimal treatment are still subject of doubts and debates4, 10, 11.
Etiological agents considered more frequent in community-acquired otitis, rhinosinusitis and bronchitis/pneumonia are Pneumococo and Haemophilus influenzae, responsible for the vast majority of cases, whereas in pharyngotonsillitis, Streptococcus pyogenes continues to be the most important agent.
Many therapeutic regimens have been used throughout the years for the treatment of these affections, especially through empirical use of antibiotics3, 4, 11, 13.
Figure 4. Final evaluation of treatment according to the person responsible for the patient.
Figure 5. Final evaluation of treatment according to research physician.
In the present study, we tried to evaluate the clinical efficacy in the treatment of community-acquired acute otitis media, rhinosinusitis and pneumonia using Broncho-Vaxom® (3.5mg/day for a 10-day period, in three consecutive months). Although it is an open non-controlled study, previous studies with control/placebo groups have consistently shown the significant clinical improvement with the use of BronchoVaxom® 1, 15.
Out of 792 patients that joined the treatment with Broncho-Vaxom®, 780 completed it according to medical prescription, showing a high degree of compliance to the proposed treatment.
The vast majority of children included in the project, regardless of the kind of respiratory affection, presented a clear reduction in the number of infections; moreover, there were other improved parameters, such as longer interval between infections and reduction of duration and severity of infections.
Subjective evaluation of efficacy, both by responsible people and physicians, was considered excellent or good in more than 90% of the cases.
Broncho-Vaxom® also proved to be a safe drug, presenting few side effects and most of them of mild severity, related to GI symptoms (diarrhea, nausea, vomiting), and it did not make patients interrupt treatment.
The data presented show that Broncho-Vaxom® may be an excellent adjuvant and a powerful weapon in our therapeutic and preventive arsenal to manage community-acquired respiratory infections in children, because the percentage of patients with clinical improvement reached almost 100%.
New multicenter double-blind studies with control groups and longer follow-up should be conducted in order to complement this initial project.
REFERENCES
1. BENTES, R. S. A. - Broncho-Vaxom prevents recurrences of respiratory tract infections. Acta Ther., 7 373-389, 1981. (11, zagar)
2. BERMAN, S. - Otitis Media in Children. New Engl J. Med. 332: 1560-5, 1995.
3. DAGAN, R.; FLISS, D.; LEIBOVITZ, E.; ABRAMSON, O.; GREENBERG, D.; LANF, R.; GOSHEN, S.; YAGUPSKY, P.; LEIBERMAN, A. - Antibiotic treatment in acute otitis media: "in vivo demonstration of antibacterial activity". CM13: 43-48, 1997.
4. DONOWITZ, G. R.; MANDELL, G. L. -Acute Pneumonia. In: Mandell, G. L.; Bennett. J. E.; Dolin, R. (eds)-Principles and Practice of Infectious Diseases. 4ª ed., Churchill Livingstone, NY USA, 1995 pp 619-637.
5. EMMERICH, B.; PACHMANN, K.; MILATOVI, D.; EMSLANDER, H. P. - Influence of OM-85 BV on different humoral and cellular immune defense mechanisms of the respiratory tract Respiration, 59; 19-23, 1992.
6. ENDO, L. H.; CARVALHO, D. S.; SAKANO, E.; BARACAT, E. C.; REIS, M.; MORASS DE OLIVEIRA, U. - "Children Palatine Tonsils Bacteriology: a Comprehensive View". Rev. Bras. Otorrinolaringol., 64(6), 1998.
7. HOWIE, U. M.; DILLARD, R.; LAWRENCE, B. - "In vivo" sensitivity test in otitis media: efficacy of antibiotics. Pediatrics 75: 8-13, 1985.
8. JOHNSON, C. E.; CARLIN, S. A.; SUPER, D. M.; REHMUS. J. M.; ROBERTS, D. G.; CHRISTOPHER, N. C.; WHIWELL. J. K.; SHURIN, P. A. - Cefxime compared with amoxicillin for treatment of Acute Otitis Media. J. Pediatr., 119: 117-122, 1991.
9. MAUEL, J. - Macrophage activation by OM-85 BV. Respiration, 59: 14-18, 1992.
10. PECHERE, J.; GARAU, J.; GEHANNO, P. - Current trends in the antibiotic therapy of uncomplicated acute otitis media in children: concluding remarks. CMI: 73-74, 1997.
11. PIGNATARI, S. S. N.; WECKX, L. L. M.; LOZOV, L; FARHAT, C. K. - Estudo Aberto do Cefprozil no Tratamento de Infecções Comunitárias do Trato Respiratório Inferior; Sinusite Aguda e Otite Media em Crianças. Ped. Mod.24: 201-207, 1998.
12. PUIGDOLLERS. J. M.; RODES, S. G.; HERNANDEZ del REI, I:; BARRUFET, T. M. T.; TORROELLA, J. J. - Immunoglobulin production in man stimulated by an orally administered bacterial lysate. Respiration, 40. 142-149, 1980. (10, Zagar)
13. WALD, E. - Management of Acute Bacterial Sinusitis in Children. in: Johnson, J., Yu, V. (eds)- Infectious Diseases and Antimicrobial Therapy if the Ears, Nose and Throat. W. B. Saunders, Philadelphia, USA. 1997 pp333-340.
14. WILBER, R. B.; DOYLE, C. A.; DURHAN, S. J. - Safety profile of Cefprozil. Clin. Infect. Dis. 14:264-71, 1992.
15. ZAGAR, S.; LOFLER-BADZEK, D. - Broncho-Vaxom® in Children with Rhinosinusitis: A Double blind Clinical Trial - ORL, 50. 1-8, 1988.
* Collaborating Professor, Discipline of Pediatric Otorhinolaryngology at UNIFESP-EPM. Responsible for the Service of Pediatric Otorhinolaryngology at COF - Hospital Professor Edmundo Vasconcelos.
** Full Professor and Head of the Discipline of Pediatric Otorhinolaryngology at UNIFESP-EPM.
*** Collaborating Professor, Discipline of Pediatric Otorhinolaryngology at UNIFESP-EPM. Director of Centro de Otorrinolaringologia a Fonoaudiologia de São Paulo - Hospital Professor Edmundo Vasconcelos.
Address for correspondence: Dra. Shirley S. N. Pignatari - Rua Vergueiro 3645 - Apto. 808 - Vila Mariana - 04101-300 São Paulo/ SP - Tel: (55 11) 5579-4502 -
Cellular: (55 11) 9905-6653-E-mail: pigna@terra.com.br
Article submitted on September 28, 2000. Article accepted on October 6, 2000.