Year: 2003 Vol. 69 Ed. 6 - (15º)
Artigo Original
Pages: 824 to 828
Frequency of positive skin tests for airborne allergic agents
Author(s):
Ricardo Godinho1,
Márcio Lanza,
Andréa Godinho,
Anderson Rodriques,
Tânia M.L. Assiz
Keywords: allergic rhinitis, allergy, epidemiology, frequency, skin test
Abstract:
Medical literature has reported on the increase in the prevalence of nose diseases, and allergic rhinitis still leads the statistics. The skin test is important in the differential diagnosis of nose diseases and to determine the sensitivity pattern of the population in order to establish measures to reduce exposure. This is fundamental for adequate handling allergic rhinitis. Study design: Transversal cohorte. Material and Method: In order to determine the frequency of positive skin tests for specific airborne allergic agents, we assessed medical records of 398 patients with nasal symptoms suggestive of allergy or other otolaryngologic complaints which required skin testing to aid in the diagnosis. Results: The most frequently found allergic agents were house dust (74,9%), dermatophagoides farinae (58,4%) and mold (36,5%), followed by dog hair (32,7%), grass (22,4%), cat hair (16,9%), feathers (16,4%) and wool (15,6%). Only 3,5% were positive for lovebird feathers and 1,5% for flowers. 39 patients (9,8%) had negative skin tests, 10 (2,5%) had reactions to the solution extract and 8 (2%) did not react to histamine. We did not have complications in performing the test. Conclusion: House dust was the most frequently found allergic agent. Skin tests must be performed based on the clinical history, and their results may guide us towards the correct diagnosis and treatment of nose diseases.
![]()
INTRODUCTION
In Brazil, Rizzo et al. (1995) found a prevalence of 32% of allergic rhinitis in a study comprising three regions of the country. In an international cooperative study 1, also conducted in Brazil, the prevalence ranged from 7.9% in Curitiba to 31.8% in Sao Paulo. The conduction of this study in Itabira 2, country area of the state of Minas Gerais, demonstrated a prevalence of 9.3% between 6 and 7 years and 11.2% between 13 and 14 years subjects. The main airborne allergic agents responsible for allergic rhinitis are mites, fungi, pollen, epithelia and animal saliva 3, 4. Molecules of airborne allergic agents are normally water-soluble and can easily disperse in the air, but their exact composition is still unknown, but normally comprises carbohydrates and proteins. They are capable of penetrating in the mucosas and to react with IgE antibodies linked to mast cells they need molecular complexity to trigger a series of reactions that result in allergic symptoms. The diagnosis of allergic rhinitis is most of the times clinical, but sometimes it is necessary to make use of nasal cytology and skin test for differential diagnosis with other rhinitis forms (non-allergic eosinophil, vasomotor, irritative, occupational, associated with systemic diseases) 5. Prick Test is the most frequently used skin test because it is easy to perform, has very few adverse effects (risk close to 0.05%), and it is not painful. The skin test is very important in the differential diagnosis of nasal diseases and for the determination of a sensitization pattern of the population in order to promote measures to reduce exposure, which is essential for the appropriate treatment of allergic rhinitis.
MATERIAL AND METHOD
In order to determine the frequency of positive responses to skin test by specific airborne allergic agents, we assessed the medical charts of 398 patients with nasal symptoms suggestive of allergy and other ENT complaints that required the test for diagnostic clarification. The patients selected were from a private clinical setting in the city of Sete Lagoas, a reference in the region that has about 500,00 inhabitants, in the countryside area of the state of Minas Gerais (70 Km from the capital city, Belo Horizonte).
The present study complied with the ethical standards dictated by the Helsinki Convention.
The criteria for exclusion were: skin lesions on the forearm surface (eczema, wounds, prurigo, dermatographism, others) and previous history of anaphylactic shock. Classic anti-histamine drugs were interrupted for 5 days and new generation drugs for 7 days. Astemizole was interrupted for 6 weeks before the test. The use of systemic or topical corticoid for prolonged time was assessed (> 7 days). Tricyclic antidepressants were interrupted for 7 days before the test and H2 antagonists only 1 day before the test 6.
PRICK TEST TECHNIQUE
The patient was instructed about the test routine and its objectives. The medial forearm surface was evaluated to exclude dermal damage and then cleaned with alcohol 70%. The extract of allergens (one single drop) was placed using a drop dispenser at about 2cm from the arm, in a pre-determined sequence (1st row: histamine, home dust, dermatophagoid, dog hair, cat hair, sheep wool, and 2nd row: extract diluting, bird feather, grass, mold and flowers). We used PUNTOR® (plastic device that limits the level of penetration into the skin) for each allergen. After 3 minutes, we removed the excessive extract with a paper towel, preventing contamination of neighboring tests. We read the reactions 15 to 20 minutes after puncture. In the absence of papule in the test with extract diluting agent, the presence of papule larger than 3mm indicates positive test.
We used allergic extracts produced by the pharmaceutical company MERK in Brazil: home dust (5000PNU/ ml), Dermatophagoides pteronyssinus (1500PNU/ ml), dog hair (5000PNU/ ml), cat hair (5000PNU/ ml), sheep wool (5000PNU/ ml), grass, fungi I (A. alternata - C. herbarum - C. globosum) (5000PNU/ ml), flowers (10000PNU/ ml) and lovebird feathers (5000PNU/ ml).
STATISTICAL ANALYSIS
The database was digitalized using software EPI-INFO 6.0 and consistency and analyses were conducted with software SPSS 8.0.
RESULTS
We assessed the frequency of positive skin tests for airborne allergic agents in 398 patients with ENT symptoms. Only four children (1%) were aged 2 years, and 14 patients (4.2%) were over 60 years (Table 1).
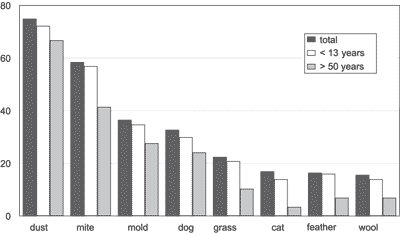
The frequency of positive skin test involving all age ranges, the group patients aged 2 to 13 years and the other one with patients older than 50 years is shown in Graph 1. Home dust (74.9%), dermatophagoid (58.4%), and mold (36.5%) were the most frequent allergens., followed by dog hair (32.7%), grass (22.4%), cat hair (16.9%), feathers (16.4%) and sheep wool (15.6%). Only 3.5% had positive test for lovebird feather and 1.5% for flowers. Thirty-nine patients (9.8%) presented negative skin test. Ten patients (2.5%) presented reaction to the solution extract and 8 (2%) did not react to histamine. We did not find any complications to the test application.Table 1. Descriptive age statistics.
Graph 1. Frequency (%) of positive skin test total and age range
DISCUSSION
The specific IgE findings against an antigen indicates sensitivity to it, not always coinciding with the presence of the disease, requiring an interaction of multiple factors for its development. Skin tests should be conducted based on the clinical history, being of great use owing to its simplicity, quickness, low cost and high sensitivity. Prick Test is positive in 10 to 15% of the subjects without symptoms, who can occasionally present symptoms of allergic disease throughout the years. An asymptomatic child with positive skin test has 50% greater risk of developing rhinitis within the next 5 years. These tests have a high educational value, since patients can clearly visualize the allergic response, reinforcing the oral information and helping them assess the cause of allergies. These tests should be conducted before any drastic measures are taken, such as removal of the family pet from home. Immunotherapy should not be implemented without skin test evidence as support.
RAST or other serological tests can be indicated in case there is no tolerance to skin test, in patients with dermatographism, severe dermatitis, use of drugs that can influence the skin response to antigen-antibody reaction, in need for confirmation tests and in subjects that present risk of severe reaction to the investigated allergens. Serum specific IgE measure is the most important in vitro method for the diagnosis of IgE-mediated hypersensitivity, but it has proved to be less sensitive than skin tests. Serology tests are costly, does not provide immediate results and do not bring additional information when compared to skin tests.
Home dust (74.9%) and Dermatophagoides pteronyssinus (58.4%) were the most frequently identified allergens in our study. Mites are the main allergenic components of home dust and there are over 300 species that are part of dust 7. They belong to a subclass of arachnids that measure about 100 to 300 millimeters, being that the feces are the most important allergens. Dermatophagoids (bed mites) are free life microorganisms that feed from desquamated skin, which is released on the bed and also on the clothes, pillows, teddy bears and dolls made of fabric. Fungi and other substances rich in protein also serve as feeding sources. The prevalence of mites is higher in humid areas and houses. Ideal conditions for the development of mites are relative humidity of 80% and temperature above 20o C. If humidity goes below 50%, mites are dried out and die. The proteins eliminated in mites' feces are the main allergens that cause the allergic symptoms 8.
Oliveira et al. (1994) reported that the main allergen involved in the triggering of asthma episodes is home dust. According to Castro (1997), 85% of the patients with respiratory allergy reported flare of crisis when in contact with home dust; in the same study, they reported imunoallergic tests of positive immediate reading for 80% of the patients and the main antigens were: Dermatophagoides pteronyssinus, Dermatophagoides farinae and Blomia tropicalis. In Brazil, epidemiological studies have demonstrated prevalence of two species of mites Dermatophagoides pteronyssinus 9-11 and Blomia tropicalis12, which are modified depending on weather conditions, humidity, temperature and nutritional factors of the patients. Arruda et al. (1991), in Sao Paulo, demonstrated positive skin results for B. tropicalis in 78% of the cases and for D. pteronyssinus in 77%. In the south of Brazil, in which there is seasonal and characteristic weather, high amounts of mites were detected: Dermatophagoides pteronyssinus, Blomia tropicalis, Tyrophagus putrescentiae and Cheyletus mallacensis13. In Belo Horizonte the most frequent ones were D. farinae, G. domesticus and D. pteronyssinus14. In a recent study conducted in Belo Horizonte with children who had allergic symptoms, Marques (1998) found 86% positive results for mites. The positive results isolated were D. pteronyssinus (78%), D. farinae (74%), B. tropicalis (64%), E. maynei (47.3%), L. destructor (36%), T. putrescentiae (31%) and G. domesticus (23.3%). A study conducted in the Service Otorhinolaryngology, Hospital das Clínicas (HC) -UFMG15, with the same allergenic extract used in our study, found positive responses of 49.4% for D. pteronyssinus and 64.8% for home dust. The findings of that study and the results of our study demonstrate the importance of home dust and Dermatophagoides pteronyssinus in the pathophysiology of allergic rhinitis in our country.
In Brazil, the frequency of positive tests for fungi ranges from 2.2% to 33% 16 and in our study conducted in Minas Gerais, mold (36.5%) was a very frequent allergen. In Belo Horizonte, Marques (1998) found positive result of 26% for fungi (Alternaria and Hormodendrum). In the study conducted at HC-UFMG17, positive results for fungi were of 6.6%. This percentage of the population allergic to fungi is resultant from continuous exposure and the problem of poorly constructed houses (syndrome of the sick home). Fungi depend on animal or vegetal material for nourishment and high relative air humidity is essential for their development, whereas sunny days favor release of spores. Despite the fact there was no strong correlation between skin tests, RAST and bronchial stimulation tests, the role of fungi in respiratory allergy has been well defined. In general, the causes of asthma are spores, but other particles (including micelle) can have allergenic activity. Different species of fungi can cause allergies in different conditions of temperature and humidity, and there is cross reactivity between the species that belong to the same class. In Belo Horizonte, according to Faria (1997), the most constant genera in the atmosphere were Cladosporium, Penicillium and Aspergillus.
About 20% to 30% of the patients with asthma or allergic rhinitis present positive skin tests for dog hair and the same values for cat hair 4. In Belo Horizonte 18, for cat epithelium, they found positive values of 18% and for dog hair, 12.7% (cases) and 9.1% (controls), which did not show statistically significant difference (p= 0.561). In our study, the frequency of positive test for dog hair was 32.7% and for cat hair it was 16.9%. At HC-UFMG15 the authors found lower frequency of positive response for dog hair (3.3%) and for cat hair (6.6%). Hairs are not considered significant allergens, since they are not water-soluble and do not fluctuate. Proteins of saliva, urine and mites linked to hairs, however, are considered significant allergens. Children can take animal allergens in their clothes in enough quantity to trigger allergic symptoms in sensitized school peers 19, 20. Home furniture and walls also preserve allergens for a considerable period of time, even after the removal of the cat 21. Feathers (16.4%) and sheep wool (15.6%) are also considered animal antigens. Feathers present a questionable role in triggering allergy, but the contamination by mites and fungi can explain its action, which is the same case of wool-manufactured products.
CONCLUSION
Skin tests should be conducted considering the clinical history of the patient and they help make allergic patients aware of the problem. The study demonstrated that home dust, dermatophagoid and mold are the most frequent allergens, followed by dog hair, feathers, wool and cat hair. The definition of the pattern of sensitization of the population in a specific region is important, since it makes possible the adoption of specific measures that will reduce the contact with the most frequent airborne allergic agents and, consequently, the allergic manifestations.
ACKNOWLEDGEMENT
The authors would like to thank Dr Maurício Mattar for his important contribution.
REFERENCES
1. Naspitz CK Epidemiology of allergic respiratory diseases in Brazil. In: Oehlinga K, Huerta López JG. Progress in allergy and clinical immunology; Proceedings of the XVIth International Congress of Allergology and Clinical Immunology. Cancún, Mexico: Hogrefe & Huber Publishers; 1997. 90-3.
2. Werneck GAF. Prevalência e fatores de risco para asma e outras doenças alérgicas em escolares de Itabira, MG, Brasil (ISSAC Survey). Cidade do México, 1995 (Tese de Mestrado em Ciência da Saúde Ambiental - Escola Nacional de Saúde Pública do México).
3. Castro FFM. Rinite Alérgica - Modernas Abordagens para uma Clássica Questão. 1ª. São Paulo: Lemos Editorial e Gráficos; 1997. 295p.
4. Mygind N. Alergia. Um Texto Ilustrado. 1ª. Rio de Janeiro: Revinter; 1993. 481 p.
5. Jacobs RL Non-allergic chronic rhinitis syndromes. Immunol Allergy Chin North Am 1987; 7:93.
6. Miller J, Nelson HS. Suppression of immediate skin tests by ranitidine. J Allergy Clin Immunol 1989; 84:894-9.
7. Ledford DK. Indoor allergens. J Allergy Clin Immunol 1994; 94:327-30.
8. Greif Fl, Andersson M, Svensson J et al. Absorption across the nasal airway mucosa in house dust mite perennial allergic rhinitis. Clin Physiol and Functional Imaging 2002; 22(1): 55-7.
9. Fain A. Allergies respiratoires produites par un Acarien (Dematophagoides pteronyssinus) vivant dans les poussières des habitations. Bull Acad R Med Belg 1966; 6(6):479-500.
10. Negreiros EB, Filarid C, Tebyriça JN et al. Alergia ao pó de casa; estudo comparativo entre os extratos totais do pó de casa - Dermatophagoides pteronyssinus e Dermatophagoides farinae em pacientes do Rio de Janeiro. Folha Med 1975; 71:385-8.
11. Flechtmann CHW, Rosa AE. Estudo da fauna acarina da poeira domiciliar no Brasil. Rev Bras Alergia Imunol 1980; 2: 91-4.
12. Medeiros JRM, Figueiredo JP. Sensibilização a aeroalérgenos em indivíduos com asma brônquica e/ou rinite crônica em Salvador, Bahia. Rev Bras Alergia Imunopatol 1997; 20(4):143-54.
13. Rosario NA, Baggio D, Suzuki MM et al. Ácaros na poeira domiciliar em Curitiba. Rev Bras Alergia Imunopatol 1992; 15(5).
14. Moreira NS. Acarinos Pyroglyphidae e outros Sarcoptiformes em amostras de pó domiciliar em Belo Horizonte, MG. Belo Horizonte, 1975 (Tese de Mestrado em Zoologia e Parasitologia - Instituto de Ciências Biológicas da UFMG).
15. Gonçalves DU, Pedrosa BF, Viana LÍNGUA, Guimarães RE, Becker HMG, Becker CG. Citologia nasal no diagnóstico de rinite alérgica. Rev Bras de Otorrinolaringologia 1993; 59:272-5.
16. Faria A. Aspectos ecológicos e clínicos da flora micótica anemófila de Belo Horizonte. Belo Horizonte, 1997 (Tese de Doutorado em Microbiologia e Imunologia - Faculdade de Medicina da Universidade Federal de Minas Gerais).
17. Howarth P.H Allergic rhinitis: a rational choice of treatment. Respir Med 1989; 83: 179-188.
18. Marques MC. Sensibilização a aeroalérgenos em crianças e adolescentes com manifestações alérgicas respiratórias. Belo Horizonte, 1998. 127p (Dissertação de Mestrado - Fac. Medicina UFMG).
19. Munir AKM, Einarsson R, Shou D, et al Allergens in school dust: The amount of the major cat (Fel d l) and dog (Can f l) allergens in dust from Swedish school is high enough to cause perennial symptoms in most children with asthma who are sensitized to cat and dog. J Allergy Clin Immunol 1993; 91:1067-74.
20. Ronmark E, Perzanawski M, Lundback B et al. High levels of cat and dog allergens in schools in Northem Sweden - In Proceedings of XVIth International Congress of Allergology and Clinical Immunology. Cancún: Hogrefe & Huber Publishers; 1997.
21. Wood RA, Mudd KE, Eggleston PA. The distribution of cat and dust mite allergens on wall surfaces. J Allergy Clin Immunol 1992; 89:126-30.
1 Centro Mineiro de Otorrinolaringologia Pediátrica - Belo Horizonte MG.
Instituto de Otorrino de Sete Lagoas - Sete Lagoas MG.
Address correspondence to: Rua Joaquim Coura, 347 Bairro Santa Helena 35700-409 Sete Lagoas MG.
Tel/fax (55 31) 3772-2121 - E-mail: ricg@uai.com.br
Study presented at I Congresso Triológico de Otorrinolaringologia, November 14-18, 1999, Sao Paulo, Brazil.
Article submitted on May 01, 2003. Article accepted on September 25, 2003.