Year: 2001 Vol. 67 Ed. 5 - (1º)
Artigos Originais
Pages: 604 to 610
Vestibular and cochlear neurectomy in the alive guinea pig: an occipital approach
Author(s):
David, Ricardo 1,
Colafemina, José Fernando 2
Keywords: neurectomy, guinea pig, vestibule, cochlea
Abstract:
Study design: Experimental. Aim: This paper describes a new surgery neurectomy technique to the VIII pair in Guinea pig alive. This is a full boned way, except for the access to the bone itself, what implies in minor damage to important structures of the animal. The animal recuperation is pretty good for later studies of the vestibular and cochlear system, and all its references to inner, middle and external ears. Conclusion: Even though the precision and control necessary, because of the neighbourhoods vital structures, it shows enough availability from the practice sight, and much less invasible and damage than other surgeries with the same finality. It allows imitaciometry, bera, vemp, stapediun and tympanic tensor reflexes and others (physicals or physioelectric).
![]()
INTRODUCTION
Yves Cazals et al. (1980)2 recorded signs of the auditory cortex and central nervous system after complete destruction of Corti's organ through administration of amicacyn, an aminoglycoside antibiotic. Despite the alteration of signs, it was possible to observe that recordings were similar to those reported by other authors when studying the stimulated vestibular system.
Although it had later been discovered that such antibiotics affect also the saccule, which is the most sensitive portion of the Corti's organ, the study attracted attention to the participation of the vestibular system in reflexive hearing.
Later, Wit and Bleeker (1982)11 recorded reflexes that probably originated from the vestibular system after the surgical destruction of the cochlea of pigeons. The stimuli, however, were collected from cephalic rotation and not sound stimuli.
The following year, Saidel and Popper (1983)10 studied in Monostariophysine teleosts fish the presence of saccule of hearing cells, possibly responsible for localizing the source of external noises.
Hildeshiner (1987)7 et al. described a surgical route for section of the superior vestibular nerve of guinea pig. Through the subarcuate fossa, a tubular structure of the temporal bone, it was possible to reach the nerve in the region of the inner acoustic canal. This tubular structure normally accommodates a dorsal and ventral floccular extension of the cerebellum.
This technique has interesting characteristics, such as: reduced cranial opening, little sacrificed cerebellar tissue, partial isolation of the operative field, in addition to showing a direct guide to the inner acoustic canal and the vestibular nerve.
Colebatch et al. (1992)4 recorded VEMP before and after unilateral neurectomy in two human beings, and they noticed the disappearance of VEMP waves N13 and P23. They also observed that waves N34 and P44 were still present, but with reduced amplitudes, and they concluded that these waves were possibly Originated from the cochlea.
This hypothesis generated controversy for some researchers - Ferber Viart et al. (1992)3-, who studied VEMP in the trapezium muscle. Ferber Viart (1992)3 divided groups of normal volunteers, patients with cochleovestibular dysfunctions, patients with only vestibular or only cochlear dysfunctions. He noticed that in the trapezius the reflex persisted in all groups, differently from the theory by Colebatch (1992)4. It is believed today that Ferber Viart (1992)3 had not considered the proximity of the recording sites of right and left trapezius muscles, suggested by the interference of signs from muscles too close one from the other.
Murofushi et al. (1995)9 recorded cell level of anesthetized guinea pigs by counting the neurons that responded to the high intensity sound stimuli of the saccule. The method, however, was extremely invasive and incompatible with survival after the end of the experiment. In order to reach the vestibular nerve, the occipital bone was removed, together with part of the cerebellum, including the paraflocculus, which was aspirated to enable visualization of the vestibular nerve, as described by Curthoys (1981)5 and (1982)6.
Murofushi (1996)8 studied the presence of reflexes in the vestibular nucleus of guinea pigs and presented the accepted proposal today that the shortest way to a reflex is an arch of three neurons, that is: primary vestibular afference, vestibulo-cochlear neuron and myogenic motoneuron.
Barbara et al. (1999)1 proposed a surgical technique that consisted of suboccipital access (retrosigmoidal) to the vestibular nerve, through the posterior-medial aspect of the temporal bone. The characteristics of this method were minor retraction and removal of cerebellar tissues, which did not affect postoperative recovery nor hearing level. The technique is similar to Curthoys's (1981)5 (1982)6.
In order to investigate and find out the existence of reflexes in the construction of a mathematical model of VEMP, it was noticed that we needed to confirm the origin of the auditory reflexes coming from the saccule.
Another alternative to the problem is surgical section of the nerve, a very delicate and limited task because of its anatomical and physiological positioning.
In human beings, the 8th nerve emerges from the auditory system connecting the vestibular portion to the cochlea inside the ear. Differently from guinea pigs, in which the nerves get together inside the brain.
In guinea pigs, the purpose has been achieved by intracranial routes, by routes that pass between the skull and the encephalic mass, comprising very delicate paths, and requiring much attention and care in incisions, in order to meet the goals without affecting the brain, which normally lead to very significant sequelae.
In guinea pigs, as mentioned before, the nerves cross separately the ossicle wall and only get together in the inner portion of the skull, immediately before it reaches the brainstem. This fact enabled the access to both two portions of the nerve independently, through an entirely bony path.
We describe next the methodology of the surgery and postoperative results.
MATERIAL AND METHOD
We sacrificed six animals of different ages and weights in order to make anatomical and anatometric comparisons.
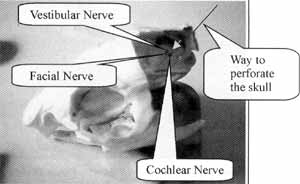
Figure 1. Anterior view of the skull, perforation in detail.
Figure 2. Middle line, through which 8th nerves are visualized
Figure 3. Skull section to visualize the 8th nerve.
The animals were then dissected for the location of vestibular, cochlear and facial nerves, in studies followed by the literature about anatomy.
In the sequence, the whole structure of organic tissues was removed, followed by cooking of the heads for about 20 hours for softening and detachment of tissues.
After the removal of soft tissues, the heads were placed in an oxygenated water bath for 24 hours, to fully clean and whiten the bony structure.
With the clean bony framework, we conducted bone perforation, a very easy task under the circumstances, since the soft tissues had been removed. It is possible to visualize through the orifice of the bone marrow passage that the emergence of cochlear, vestibular and facial nerves, was on the internal wall of the skull (Figure 3), visually guiding the perforation.
Figure 1 illustrates well the access with a metallic needle indicating the necessary inclination to reach the cochlear nerve. The circles show one of the paths mentioned by other authors, such as Murofushi (1995)9, (1996)8; Curthoys (1981)5, (1982)9; Barbara (1999)1.
Figure-2 illustrates with the arrow the line from where we can see the perforation path to reach the 8th nerve, through the clean bone. Through the orifice, we could see three perforations in the internal wall of the skull, comprising facial, cochlear and vestibular nerves (Figure 3).
The inclination of the drill had to be well defined, as well as the depth of the perforation and the starting point for the skull perforation. The anatomical study of the six sacrificed guinea pigs enabled the definition proportions based on size of the skull, which provided different ways to reach the nerve, without compromising the other structures.
Figure 4. Calculation of perforation, depth to reach the 8th nerve.
Figure 5. Guiding device to direct the perforation angle of the surgery.
Figure 6A. Trichotomy and Y incision. Figure 6B. Scalp of temporal bone.
Figure 3 shows a cranial section through which we can see the orifices of the 8th nerve nervous bundles, coming from the cerebellum. The arrow points to the orifice made by the present surgery.
Figure 7. Mean for calculation of perforation depth.
Figure 8. Position of perforation. After placement of electrode or complete section of the nerve, we conducted careful cleaning to remove bone particles from the live tissue, and finally, the surgical suture.
Figure 9. Final surgical suture.
Once we had the six cleaned and perforated skulls, we started the anatometric study of the pieces, which defined parameters for in vivo surgery in the guinea pigs, defining starting points of the perforations, inclination angles of the drills, its diameter and depth to reach the target nerve. It is important to note that through this technique, it is possible to reach independently cochlear and vestibular nerves. Facial nerve is located behind the auditory nerves (in this path), making it difficult to reach it without compromising the cochlear and vestibular nerves.
The beginning of the perforation was on the medial portion of the mastoid process, in the continuity line with lambdoid suture.
The depth of the perforation was calculated by measuring the distance between the upper frontal incisors and the anterior extremity of the skull, occipital scale, and by dividing the result by 6.91 (Figure 4).
The arrow in Figure 4 shows the extremity that should determine the distance used to calculate the perforation depth.
One of the angles used to introduce the drill is represented in Figure 4, where it is possible to see the drill inserted in the orifice. In order to reach the cochlear nerve, the drill should point to the center of the external acoustic canal. To reach the vestibular nerve, the drill should also point to the external auditory canal, but tangentially to the internal superior portion of it.
To define other inclination angles, it was necessary to construct a small mechanical device to serve as a guide of the perforation, although practice may exclude its use (Figure 5).
We used a dentist drill or bur with a diameter of 1/ 16", enough for the complete section of the cochlear nerve. To the superior vestibular nerve, we can make use of smaller drills, with one caveat: the diameter of the holder is larger than the drill itself, generating friction that may take to excessive heating of the bony wall, causing burning of the cerebellar tissues.
Description of the surgery
Male guinea pig weighting 400-600 grams were anesthetized with tyonambutal, dosed between 20 and 30mg/Kg. After head trichotomy, we applied local anesthesia on the region of the incision, dosed from 2 to 4ml of injecting xylocaine, on the incision line (Figure 6). Incision was made in Y, as shown in Figure A, which affects the tissues less.
Using a knife and a brushing spatula, we detached the scalp from the skull to clean and show the temporal bone, as illustrated in Figure 6B.
Using an inverted clamp, we maintained the working area opened.
The guinea pigs were taped to a small surgical table with head and trunk torsion and rotation resources.
A pachymeter, or another measurement instrument with the same purpose, was used to measure the distance from the upper incisors and the superior portion of the atlas (Figure 7).
After the measurement, the value was divided by 6.91 from where we learned the depth of the perforation to reach the cochlear and vestibular nerves.
We placed the drill in the medial portion of the mastoid process, in the line of lambdoid suture and marked a point to start the perforation of the right and left hemispheres.
By using the device shown in Figure 5, it is possible to guide the perforation to reach the targets using it to create the drill inclination in two of three angles.
The third angle is visually obtained directing the perforation towards the center of the external auditory canal, to the cochlear nerve, or towards the internal upper tangent of the same canal, to reach the vestibular nerve (Figure 8).
In the holder of the drill we marked the calculated depth, about 5mm.
The perforation was started at half speed to avoid overheating the bone. After the first 3 or 4 mm of perforation, the drill got into the subarcuate fossa.
At this point, with the halted drill, we positioned appropriately the perforation angles to conclude the surgery. With continuous practice, the guiding device may be abandoned. With the drill inside the subarcuate fossa, there could be an overflow of blood through the perforation, suspended after few seconds, enabling the rest of the procedure to be concluded.
This is a delicate phase, because cerebellar tissue is really close by and there are no obstacles to prevent a trauma from happening.
Once the target was reached, with a syringe, we sucked the nervous mass which had been ruptured by the tip of the drill. Much Care should be taken with the use of some collecting syringes because we should concentrate on precision to reach the nerve canal without damaging it.
Except for balance disorders or hearing loss, basic objectives of the surgery, the guinea pigs recovered well. In six successfully conducted experiments only one guinea pig died within 24 hours; three died slowly, probably because of some contamination, after one week and a half, and two remained alive.
Recovery of guinea pigs
Guinea pigs that survived over one week were autopsied and we found that the perforations made were at final stage of calcification. The other two guinea pigs were sacrificed 20 and 30 days after the surgery and we observed complete calcification of the perforations, as well as good reconstitution of the remaining tissues.
DISCUSSION
Compared to other techniques described Hildeshiner (1982)7 and Barbara, (1999)1 - this new technique has some advantages:
a) since the path is entirely bony and the structure is minimally damaged, there was no damage to any vital structures. Even if there are anatomical or physiological postoperative sequelae, then is no damage to the encephalic mass or to air other noble tissue rather than the nerves targeted by the surgery.
b) it enables a more precise and realistic study since the animal recovered levels very similar to the ones it had before the surgery. The perforated bone structure itself was recovered in approximately 2 weeks, by means of natural calcification.
c) the complete surgical time is rather short, and with some practice it is possible to complete the whole procedure is less than 40 minutes leading to less traumatism to the animal and use of fewer anesthetic agents. There are no comparative data concerning other techniques in a test using Barbara's technique (1999)1, two hours were required to complete the procedure We should take into account, however, our lack of experience with the technique.
One disadvantage of the new technique is the precision required throughout the procedure. It is necessary to study thoroughly the path that is going to be approached After study and practice, however, the technique tends to get improved by itself, reaching very high satisfaction level; and dispensing the use of the guiding device described it the present article.
CONCLUSION
The new technique presented here sheds new light into the research of vestibular and cochlear systems opening a myriad of options owing to less tissue invasion.
It is clearly one further step towards an idea neurectomy, in which no surrounding structures will be damaged.
After improvement of the technique, it is possible not only to destroy the nerve, but as shown here, to introduce electrodes to record direct signs from the vestibular and cochlear nerve, with no compromise of vital surrounding structures. Injection of neurotoxic drug directly in nervous tissues may also produce satisfactory research responses in the area.
The idea may also serve to suggest other procedures correlated or not, to be applied in different structures of the animal body aiming at reaching other types o; structures.
ACKNOWLEDGEMENT
We would like to acknowledge CAPES for them financial support and the technicians from the experiments: surgery division of Hospital das Clínicas de Ribeirão Preto for their dedicated support during the surgeries.
REFERENCES
1. BARBARA, M.; ATTANASIO, G.; PICCOLI, F.; FILIPO, R. - Vestibular Neurectomy in the guinea pig: a retrosigmoid approach. Acta Otolaryngol, Mar. 119(2):171-3, 1999.
2. CAZALS, Y.; ARAN, J.M.; ERRE, J.P.; GUILHAUME, A. - Acoustic Responses after total destruction on the cochlear Receptor: Brainstem and auditory cortex. Science. V. 210 3 oct. 1980.
3. CHANTAL, F.V.; NATHALIE, S.; CHRISTIAN, D.; ROLAND, D. Cochleovestibular afferent pathways of trapezius muscle responses to clicks in human. Acta Otolaryngol (Stockh) 118.6-10, 1998.
4. COLEBATCH, J.G.; HALMAGYI G.M. - Vestibular evoked potentials in human neck muscles before and after unilateral vestibular deafferentation. Neurology aug. 42:1635, 1992.
5. CURTHOYS I.S. - Scarpa's ganglion in the rat and guinea pig. Acta Otolaryngol, 92:107-113, 1981.
6. CURTHOYS I.S. - The response of primary horizontal semicircular canal neurons in the rat and guinea pig to angular acceleration. Exp Brain Res 47: 286-294.
7. HILDESHEIMER, M.; MUCHNIK, C.; RUBINSTEIN, M. - Surgical Approach to the superior vestibular nerve in guinea pigs. Hear Res Dec; 31(2):193-5, 1987.
8. MUROFUSHI, T.; CURTHOYS, I.S. - Response of guinea pig vestibular nucleus neurons to clicks. Exp Brain Res, 111:149-152, 1996.
9. MUROFUSHI, T.; CURTHOYS, I.S.; TOPPLE, A.N.; COLEBATCH, J.G.; HALMAGYI, G.M. - Responses of guinea pig primary vestibular neurons to clicks. Fap Brain Res 103:174-178, 1995.
10. SAIDEL, W.M.; POPPER A.N. - The saccule may be the transducer for directional hearing of Nonostariophysine Teleost. Exp Brain Res, 50:149-152, 1983.
11. WIT, H.P.; BLEEKER, J.D. - Sensitivity of the vestibular system to acoustic stimuli. Arch. Oto-Rhino-Laryngology, 234.205-211, 1982.
1 Department of Medical Physics, Faculdade de Filosofia Ciências a Letras de Ribeirão Preto. Universidade de São Paulo (USP), Faculdade de Medicina de Ribeirão Preto. Universidade de São Paulo (USP)
2 Ricardo David - R. São Tomaz de Aquino, 1004 - Jrd Francano, Franca, SP
Tel (55 16) 3721 1623 - 9122 3093 - E-mail: rdavid@dfm.ffclrp.usp.br - rdavid@sf.com.br
The present study was financially supported by CAPES.
Special citation at II Congresso Tecnológico de Goiânia.
Article submitted on April 12, 2001. Article accepted on May 10, 2001.