Year: 2002 Vol. 68 Ed. 5 - (13º)
Artigo Original
Pages: 679 to 684
Head and neck mucoepidermoid carcinoma: clinicopathologic study of 173 cases
Author(s):
Fábio R. Pires 1,
Fábio de A. Alves 2,
Oslei P. de Almeida 3,
Luiz P. Kowalski 4
Keywords: salivary glands, head and neck, parotid, palate, mucoepidermoid carcinoma.
Abstract:
Introduction: Mucoepidermoid carcinoma (MEC) is the most common malignant salivary gland tumor, however few studies have been reported in Brazilian populations. Objective: To report clinical and pathologic data from 173 head and neck MEC treated in the Centro de Tratamento e Pesquisa Hospital do Câncer A. C. Camargo in São Paulo. Study design: Clinical randomized. Material and Method: From 1953 to 1997, 173 cases of MEC were found in the files of the Centro de Tratamento e Pesquisa Hospital do Câncer A. C. Camargo. Data were obtained from the patients' records and histological review of all cases. Results: The mean age of the patients was 44 years and 93 (53,8%) were men; parotid glands were affected in 61 cases (35,2%) and intra-oral minor salivary glands in 75 (43,4%). TNM revealed 50,3% of the cases in stages I and II, and histological grading revealed 45,2%, 18,5% and 36,3% low-grade, intermediate-grade and high-grade tumors, respectively. Surgical treatment was employed in 80,3% of the cases, with neck dissection in 52 cases (30,1%), and radiotherapy in 73 (42,2%). Local recurrence, regional and distant metastasis were found in 12,7%, 9,8% and 9,2% of the patients, respectively; 5-year and 10-year overall survival rates were 70% and 60%, respectively. Conclusions: MEC affected mainly the parotid gland and the palate of adults, without gender predilection. Half of the cases were diagnosed in initial clinical stages and 64% of the tumors were low or intermediate-grade lesions. Surgery was the treatment of choice and prognosis was good.
![]()
INTRODUCTION
Mucoepidermoid carcinomas (MEC) are malignant tumors originated from the excretory ducts of the salivary glands, which affect both major and intraoral minor salivary glands in more than 90% of the cases1, 2. MEC can also be present in recovering glands of maxillary sinus, lachrymal glands, oropharynx, nasopharynx, larynx, vocal folds, trachea and lungs2. Histologically, mucoepidermoid carcinomas have been classified in three grades of malignancy (low, intermediate and high grade) and this subdivision has proved to be useful in defining therapy and prognosis of the tumors3, 4, 5. Few studies in the literature have analyzed the clinical and pathological data of large series of MEC of different sites6-10. In most cases, MEC are considered together with other malignant and/or benign tumors of the parotid or intraoral minor glands11-18.
The purpose of the present study was to discuss clinical and histology data of 173 cases of MEC treated in one institution and compare them to the international literature.
MATERIAL AND METHOD
The clinical files of the Treatment and Research Center at Hospital do Câncer A. C. Camargo, São Paulo/SP were reviewed for cases of mucoepidermoid carcinomas, adenocarcinoma, solid glandular carcinoma and mucinous carcinoma of the head and neck region treated up to the year of 1997. The histopathology diagnosis in all cases was confirmed by assessing stained sections with hematoxylin and eosin, periodical acid of Schiff (PAS) and mucicarmine, and the cases with final diagnosis of mucoepidermoid carcinomas were included in the present study.
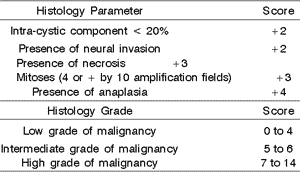
Clinical and epidemiological data, staging, location, treatment, duration of follow-up and current status of patients were collected from the medical charts. The grading of tumors in low, intermediate and high grade of malignancy was conducted based on the criteria suggested by Ellis & Auclair1 (Table 1).
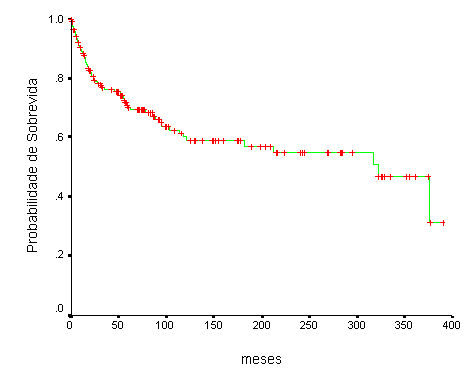
Figure 1. Survival curve (Kaplan-Meier) of 173 cases of mucoepidermoid carcinomas treated at Hospital A. C. Camargo.
RESULTS
Between 1953 and 1997, 173 cases of MEC from the Treatment and Research Center at Hospital do Câncer A. C. Camargo were confirmed after the clinical and histology review. The man : woman ratio was 1.2:1, with 93 cases (53.8%) affecting men. The age of patients ranged from 6 to 96 years, mean age of 44 years. The mean age was greater in men (mean age of 47.3 years - ranging from 6 to 79 years) than in women (mean age of 40 years - ranging from 6 to 96 years), being that 55.5% of the cases affected patients between the 4th and 6th decades of life. The mean duration of complaint ranged from 1 to 480 months, mean of 38 months, and the most frequent symptoms were increase of local volume in 162 cases (93.6%) , pain in 69 cases (39.9%), and paresthesia in 11 cases (6.4%).
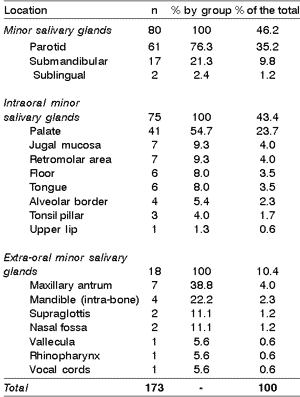
Eighty cases (46.2%) affected the major salivary glands, being 61 in the parotid, 17 in the submandibular, and 2 in the sublingual gland. Of the 93 cases (53.8%) affecting the minor salivary glands, 75 affected the intraoral glands and 18 the extra-oral glands. The distribution of 173 cases according to location of lesions is shown in Table 2. The largest diameter of tumors varied according to lesion site, and it was greater in extra-oral minor salivary glands (mean of 5.5cm - ranging from 2 to 9 cm), followed by major salivary glands (mean of 4.6cm - ranging from 1 to 16cm) and intraoral minor glands (mean of 3.6cm - ranging from 1 to 8cm). As to mobility, 74.3% of the cases were fixed or semi-fixed in the adjacent tissues, 25.7% were mobile, and invasion of one or more adjacent structures was noticed in 70.5% of the cases.
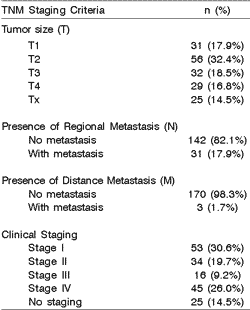
Eighty-seven cases (50.3%) were classified as T1 or T2; 31 (17.9%) and 3 (1.7%) patients presented regional and distance metastases at diagnosis, respectively. TNM staging revealed 50.3% of cases in stages I and II (Table 3).
Grading criteria showed 73 cases (42.2%) with intra-cystic component smaller than 20% of the tumor, 15 cases (8.7%) had neural invasion, 38 cases (22%) had presence of necrosis, 31 cases (17.9%) had 4 or more mitoses by 10 fields of amplification, and 87 cases (50.3%) had anaplasia. At the end of grading, 61 cases (35.3%) were classified as low grade malignant, 25 (14.5%) were intermediate grade, and 49 (28.3%) were high grade, and 38 (22%) cases were not graded owing to small tumor sampling. Considering only 135 cases submitted to histology grading, these values were 45.2% (low grade), 18.5% (intermediate grade), and 36.3% (high grade).
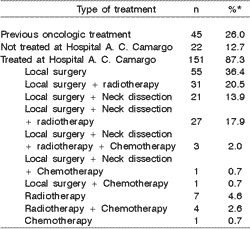
As to treatment, 45 cases (26%) had been submitted to surgical and/or radiotherapy oncologic treatment in other institutions. At the Treatment and Research Center of Hospital do Cancer AC Camargo, 151 patients (87.3%) were submitted to treatment, including surgery with local resection in 139 cases (92.1%), neck dissection in 52 (34.4%), radiotherapy in 72 (47.7%) and chemotherapy in 10 (6.6%). Twenty-two cases (12.7%) were not submitted to treatment in the hospital. The four types of treatment most commonly used included local isolated surgery or associated with radiotherapy and/or neck dissection. Table 4 shows the distribution of patients according to type of treatment performed at the hospital.
Local surgeries with margin (43.9%) and partial or total parotidectomy (40.3%) were the most common types of surgery used in 139 cases submitted to surgical procedure (Table 5). As to the 52 patients submitted to neck dissection, unilateral radical dissection (53.8%) and unilateral cervical supra-omo-hyoid dissection (40.4%) were the two most frequent techniques (Table 5). Of the 52 operated cases for neck dissection, 22 (42.3%) presented lymph node metastasis after the histology assessment.
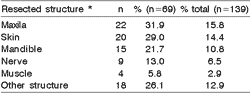
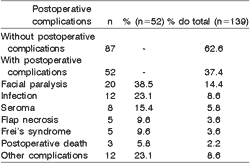
Surgical removal of tumors was associated with resection of adjacent structures in 69 cases (49.6%). Only one structure was dissected in 55 cases (79.7%) and in the remaining cases, two structures were resected. Table 6 shows the distribution of the resected structures in 69 cases. Postoperative complications such as facial nerve paralysis, seroma and infections were present in 52 (37.4%) of the 139 patients submitted to surgical procedures at the hospital. Forty-one patients (78.8%) presented only one complication and 11 (21.2%) had more than one postoperative complication (Table 7).
After treatment, 22 patients (12.7%) presented local recurrence. Regional recurrence was found in 17 cases (9.8%), being homolateral in 10 cases (5.8%), contralateral in 5 (2.9%) and bilateral in two cases (1.1%). Distance metastases affected 16 patients (9.2%) and it was more frequent in the lungs (12 cases - 6.9%) and bones (5 cases - 2.9%). Treatment of recurrence was conducted in 35 cases (20.2%) and included oral resection, neck dissection, radiotherapy and chemotherapy. The duration of follow-up ranged from 1 to 389 months (mean of 98 months) and revealed 83 patients (48%) living free from the disease, 6 patients (3.5%) were living with the disease, 45 patients (26%) had died of the disease and 16 patients (9.2%) died of other causes. Twenty-three patients (13.3%) were lost. The overall survival rate was 70% and 60%, respectively, for 5 and 10 years (Figure 1).Table 1. Criteria for histology grading of mucoepidermoid carcinomas (According to Ellis & Auclair1).
Table 2. Distribution of 173 cases of mucoepidermoid carcinomas of the head and neck according to location.
Table 3. TNM staging of 173 cases of mucoepidermoid carcinomas.
Table 4. Distribution of 173 cases of mucoepidermoid carcinomas of the head and neck according to treatment employed.
* The percentage of each type of treatment was expressed based on the 151 patients submitted to treatment at the Hospital.
Table 5. Type of local surgery and neck dissection performed in patients with mucoepidermoid carcinomas surgically treated at Hospital A. C. Camargo (n=139).
* Six patients were submitted to more than one type of Local surgery; ** One patient was submitted to more than one type of Neck dissection.
Table 6. Distribution of frequency of each resected structured adjacent to the tumors in relation to the total number (n=69) and total of patients submitted to surgical procedures at Hospital A. C. Camargo (n=139).
* In 14 of the 69 cases (20.3%). more than one adjacent structure was resected.
Table 7. Distribution of frequency of postoperative complications concerning their total (n=52) and the total number of patients submitted to surgical procedures at the Hospital (n=139).
DISCUSSION
MEC normally affects major and intraoral minor salivary glands, however, it has been identified associated with a large number of other glandular epithelium in the region of the head and neck, such as the recovery of the maxillary sinus, nasopharynx, oropharynx, vocal folds, larynx, trachea, lachrymal gland and thyroid2, 19. Most of the studies restrict the assessment of MEC to specific sites, hindering actual statistics of tumor incidence in different locations5, 14, 15. Our results confirmed that major and intraoral minor salivary glands are the preferable sites for MEC, but we would like to point out that other minor glands can be affected in up to 20% of the cases5, 10, 19.
MEC affect young adults in special, with no gender preference, and are normally manifested as an increase in volume of slow evolution, normally asymptomatic, but that can be occasionally associated with superficial ulceration, pain and paresthesia 1, 3, 10, 15. In our cases, about 40% of the patients presented pain at the time of diagnosis. Our results showed that the tumors seem to affect more advanced aged men than women, a finding not reported in the literature2, 13.
The location of tumors showed that parotid was the most frequent site, followed by palate and submandibular gland. Considered as a whole, the incidence of major glands and intraoral minor glands was similar, in accordance with the literature1, 2, 6, 8, 9, 11. As to size of tumors, MEC affecting extra-oral minor salivary glands presented the largest mean of diameter, especially of antrum-maxillary tumors, which can take large dimension before it causes patients' symptomatology.
As to staging, 50% of the cases presented initial consultation in TNM clinical stages I and II, below those reported in the world literature6, 8, 10, but similar to findings in other Brazilian publications8. Despite the fact that 50.3% of our cases presented stages T1 ands T2, 74.3% of the tumors were presented as fixed or semi-fixed, what can justify the unfavorable diagnosis of some cases even in initial stages, owing to substaging. Minor glands present tumors in more advanced stages than major glands, as reported in the literature9. In general, half of the cases presented as initial T stages (T1 and T2), 17.9% presented regional compromise and 1.7% had distance metastases, figures close to those reported in the literature6, 9.
Histopathology diagnosis of mucoepidermoid carcinomas is based on evidence of three cell types (mucosa, intermediate and squamous cells) organized in inlets, plates and cystic formations1. Cytoplasm positive response to PAS and mucicarmine or Alcian blue is important to confirm the mucoid nature of the cell material stored and secreted by tumor cells. Histology grading of mucoepidermoid carcinomas usually considers the tumor arrangement, the degree of differentiation and anaplasia of tumor cells and interrelation between the three cell types5. Various classifications have been proposed and grading is normally separated by low grade tumors, intermediate and high grade malignant tumors3, 6, 7, 8, 20. The criteria suggested by Auclair et al.3, Ellis & Auclair1, Ellis et al.2 and Goode et al.5 for grading of mucoepidermoid carcinomas of major and minor salivary glands is based on five histology parameters that are easy to observe at optical microscopy, facilitating the distinction between the three histology types. However, this one, as well as others classifications, still have limitations concerning reproducibility of some parameters since they include subjective potential of assessment, especially of the degree of anaplasia of the tumor3. Our results showed that, similar to the literature2, 6, 8, 9, 10, 21, 22, most tumors present low or intermediate grade of malignancy, what contributes to a favorable prognosis of mucoepidermoid carcinomas.
Wide local resection, associated or not with neck dissection, and occasionally postoperative radiotherapy is the preferred treatment regimen for mucoepidermoid carcinomas1, 5, 6, 9, 10, 21, 22. The resection of adjacent structures is indicated in cases in which there was compromise detected previously or during the surgery. It was necessary to perform widened resections in half of the cases treated by surgery. Neck dissection is indicated in cases in which there is clinical evidence of regional metastasis, advanced clinical staging or high histology malignancy grade1, 6, 10, 12. In about half of the cases in which neck dissection was performed we detected neoplasm impairment in the histology.
The rates of local recurrence (12.7%), regional recurrence (9.8%) and distance metastases (9.2%) found in our cases were within the range reported in the literature2, 3, 5-10, 22. Th final status of patients revealed that 5-year overall survival was 70%, highlighting the relatively favorable behavior and the good prognosis of mucoepidermoid carcinomas, when compared to other salivary gland tumors7, 8, 21.
CONCLUSIONS
Head and neck mucoepidermoid carcinomas affect especially adults on the 4th and 6th decades of life, with no gender preference. The incidence is similar in major and minor salivary glands, involving especially the parotid and the palate. Most tumors are low or intermediate malignant grade and local surgical treatment was used in about 80% of the cases, complemented by neck dissection and radiotherapy in 35% and 48% of the cases, respectively. Overall survival was 70% and 60%, respectively, for 5 and 10-year periods, confirming the good general prognosis of mucoepidermoid carcinomas.
ETHICS COMMITTEE
The research protocol included in this study was approved by the Research Ethics Committee of the Treatment and Research Center of Hospital do Câncer A. C. Camargo, São Paulo/SP.
REFERENCES
1. Ellis GL, Auclair PL, Gnepp DR. Surgical Pathology of the salivary glands. Major Problems in Pathology Series. Volume 25. Philadelphia: WB Saunders Company; 1991.
2. Ellis GL, Auclair PL. Tumors of the Salivary Glands. In: Armed Forces Institute of Pathology. Atlas of Tumor Pathology. 3rd Series, Fascicle 17. Washington; 1996.
3. Auclair PL, Goode RK, Ellis GL. Mucoepidermoid carcinoma of intraoral salivary glands. Cancer 1992;69:2021-2030.
4. Cardoso WP, Denardin OV, Rapoport A, Araujo VC, Carvalho MB. Proliferating cell nuclear antigen expression in mucoepidermoid carcinoma of salivary glands. São Paulo Med J 2000;118:69-74.
5. Goode RK, Auclair PL, Ellis GL. Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer 1998;82:1217-1224.
6. Brandwein MS, Ivanov K, Wallace DI, Hille JJ, Wang B, Fahmy A, Bodian C, Urken ML, Gnepp DR, Huvos A, Lumerman H, Mills SE. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol 2001;25:835-845.
7. Evans HL. Mucoepidermoid carcinoma of salivary glands: a study of 69 cases with special attention to histologic grading. Am J Clin Pathol 1984;81:696-701.
8. Nascimento AG, Amaral ALP, Prado LAF, Kligerman J, Silveira TRP. Mucoepidermoid carcinoma of salivary glands: a clinicopathologic study of 46 cases. Head Neck Surg 1986;8:409-417.
9. Plambeck K, Friedrich RE, Bahlo M, Bartel-Friedrich S, Klapdor R. TNM staging, histopathological grading, and tumor-associated antigens in patients with a history of mucoepidermoid carcinoma of the salivary glands. Anticancer Res 1999;19:2397-2404.
10. Spiro RH, Huvos AG, Berk R, Strong EW. Mucoepidermoid carcinoma of salivary gland origin: a clinicopathologic study of 367 cases. Am J Surg 1978;136:461-468.
11. Chinellato LEM, Marquez IM, Fleury RN, Quevedo FC. Estudos da prevalência dos tumores de origem epitelial de glândulas salivares em Serviços de Anatomia Patológica das cidades de Bauru e Jaú (Estado de São Paulo, Brasil). Rev Fac Odontol Bauru 1994;2:45-51.
12. Franzi AS, Carvalho MB. Carcinoma mucoepidermóide avançado das glândulas salivares. Rev Bras Cancerol 1997;43:273-280.
13. Kusama K, Iwanari S, Aisaki K, Wada M, Ohtani J, Itoi K, Hanai K, Shimizu K, Komiyama K, Kudo I, Moro I. Intraoral minor salivary gland tumors: a retrospective study of 129 cases. J Nihon Univ Sch Dent 1997;39:128-132.
14. Lopes MA, Kowalski LP, Santos GC, Almeida OP. A clinicopathologic study of 196 intraoral minor salivary gland tumors. J Oral Pathol Med 1999;28:264-267.
15. Loyola AM, de Araujo VC, de Sousa SOM, de Araujo NS. Minor salivary gland tumours: a retrospective study of 164 cases in a Brazilian population. Oral Oncol Eur J Cancer 1995;31B:197-201.
16. Rapoport A, De Andrade Sobrinho J, Brasilino De Carvalho M, Magrin J, Fava AS. Cancer of the parotid gland. Int Surg 1981;66:243-246.
17. Rapoport A, Carvalho MB, Fava AS, Góis Filho JF, Chagas JFS, Kowalski LP, Kanda JL, Sobrinho JÁ, Ferreira Filho G, Cheuhen JA. Diagnóstico e tratamento das neoplasias das glândulas salivares menores: estudo de 55 casos. Rev Col Bras Cirur 1988;15:289-293.
18. Regis De Brito Santos I, Kowalski LP, Cavalcante De Araujo V, Flavia Logullo A, Magrin J. Multivariate analysis of risk factors for neck metastasis in surgically treated parotid carcinomas. Arch Otolaryngol Head Neck Surg 2001;127:56-60.
19. Spiro RH, Thaler HT, Hicks WF, Kher UA, Huvos AH, Strong EW. The importance of clinical staging of minor salivary gland carcinoma. Am J Surg 1991;162:330-336.
20. Hicks MJ, El-Naggar AK, Flaitz CM, Luna MA, Batsakis JG. Histocytologic grading of mucoepidermoid carcinoma of major salivary glands in prognosis and survival: a clinicopathologic and flow cytometric investigation. Head Neck 1995;17:89-95.
21. Hicks J, Flaitz C. Mucoepidermoid carcinoma of salivary glands in children and adolescents: assessment of proliferation markers. Oral Oncol 2000;36:454-460.
22. Ma'aita JK, Al-Kaisi N, Al-Tamimi S, Wraikat A. Salivary gland tumors in Jordan: a retrospective study of 221 patients. Croat Med J 1999;40:539-542.
1 Assistant Professor, Oral Pathology, Dental School, State University of Rio de Janeiro (UERJ), Rio de Janeiro/RJ.
2 Assistant Professor, Oral Pathology, State University of Lavras (UNILAVRAS), Lavras/MG.
3 Faculty Professor, Oral Pathology, Dental School, State University of Campinas (UNICAMP), Piracicaba/SP.
4 Head of the Department of Head and Neck Surgery and Otorhinolaryngology, Treatment and Research Center, Hospital do Câncer A. C. Camargo, São Paulo/SP.
Study conducted by the Discipline of Oral Pathology, Dental School, Piracicaba/UNICAMP and by the Department of Head and Neck Surgery and Otorhinolaryngology, Treatment and Research Center, Hospital do Câncer A. C. Camargo, São Paulo/SP.
Financial Support: Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP)
Address correspondence to: Luiz Paulo Kowalski - Departamento de Cirurgia de Cabeça e Pescoço e Otorrinolaringologia, Centro de Tratamento e Pesquisa Hospital do Câncer A. C. Camargo
Rua Prof. Antônio Prudente, 211 Liberdade 01509-900 São Paulo - SP. Tel: (55 11) 3272.5125 - Fax: (55 11) 3277.6789 - E-mail: lp_kowalski@uol.com.br
Article submitted on July 27, 2002. Article accepted on August 15, 2002