Year: 2002 Vol. 68 Ed. 5 - (8º)
Artigo Original
Pages: 645 to 652
Anatomical variations and sinusitis: a computed tomographic study
Author(s):
Marcio M. Kinsui 1,
Arnaldo Guilherme 2,
Hélio K. Yamashita 3
Keywords: anatomy, nasal cavity, turbinates, paranasal sinuses, tomography
Abstract:
Introduction: Computed tomography has been increasingly used both to identify and to evaluate anatomy variations of nasal cavities that can lead to the development of sinusitis. Purpose: The purpose of the present study is to determine the incidence of mucosal abnormalities in paranasal sinuses found in CT scans of patients with symptons of sinusitis and analyze the correlation between sinusitis and presence of Haller's cell, concha bullosa and nasal septal deviation located in middle meatus. Study Design: Clinical retrospective. Material and Method: Paranasal sinus CT scans were obtained in 150 patients aged 13 years or more, from July 1999 to October 2001. The CT scans were performed in the Department of Radiology of Universidade Federal de São Paulo - Escola Paulista de Medicina. Patients with history of skull base or sinus surgery and tumor in these regions were excluded. Results: 70% of patients present mucosal abnormalities at least in one paranasal sinus. Maxillary sinusitis were observed in 52,7% of sinus, ethmoidal sinusitis in 28,0%, sphenoidal sinusitis in 13,0% and frontal sinusitis in 8,3%. Concha bullosa was observed in 33,3% of nasal cavities, nasal septal deviation (located in middle meatus) in 23,3% and Haller's cell in 9,3%. Conclusions: The most affected paranasal sinuses were: maxillary, ethmoid, sphenoid and frontal. Correlation between sinusitis and presence of Haller's cell, concha bullosa and nasal septal deviation (located in middle meatus) was not observed.
![]()
Introduction
The exact comprehension of the anatomy of the nasal cavity, emphasizing the lateral wall of the nose and its variations, is extremely important for nasal-sinusal endoscopic surgery. Anatomical variations have been considered as predisposing factors for sinusitis and include middle concha bullosa, nasal septum deviation or spore, hyperpneumatized ethmoid bulla, paradoxical middle concha, medial or lateral deviation from the uncinate process, among others. The middle concha bullosa seems to be the most frequent anatomical variation found1.
The development of new endoscopic instruments and associated techniques enabled the creation of a new surgical method for the treatment of chronic sinusitis, aiming at restoring normal physiology by means of restoring mucociliary drainage and ventilation of paranasal sinuses2, 3, 4. Owing to that, an anatomical entity named ostiomeatal complex became extremely important both for the pathophysiological phenomenon and the surgical strategies.
The ostiomeatal complex consists of the ostium of the maxillary sinus, ethmoidal infundibulum, uncinate process, ethmoid bulla, semilunar hiatus, frontal recess, middle meatus and middle concha 5. The anatomical variations of the ostiomeatal complex have been implicated in the etiology of chronic and recurrent sinusitis and for this reason, paranasal sinuses CT scan (computed tomography), especially at coronal sections, have been routinely used in the assessing patients with sinusal symptoms2, 5. We should bear in mind that most of the times, CT scan is important not only for the diagnosis of inflammatory diseases, but also to visualize the anatomical variations, which makes it an excellent exam for surgical planning.
As a result of routine use of CT scan, we can better observe the structures and anatomical variations of the nasal and paranasal cavities, among them, the Haller cells. Initially described by Von Haller in 1803, these cells are considered the expansions of pneumatization of the ostiomeatal complex located inferiorly to the ethmoid bulla and along the medial portion of the orbit floor, related to the roof of the maxillary sinus. The first observations were made through dissections of cadavers, and up to some time ago, these structures were not valued sufficiently. The understanding of the mucociliary drainage physiology of paranasal sinuses and the development of CT scan resulted in the hypothesis that Haller cells are associated with rhinosinusitis, owing to its location close to the ethmoid infundibulum.
This study intends to assess, by means of computed tomography, the incidence of mucous thickness of maxillary, ethmoid, frontal and sphenoid sinuses in patients with symptoms of sinusitis and the occurrence of Haller cells, middle concha bullosa and nasal septum deviations located in the middle meatus and their correlations with maxillary, ethmoid and frontal sinus disease.
Material and Method
We analyzed retrospectively 150 CT scans of paranasal sinuses of subjects of both genders, conducted at the Department of Imaging Diagnosis, Federal University of São Paulo - Escola Paulista de Medicina, between July 1999 and October 2001. The exams included in the sample Presented diagnoses compatible with inflammatory sinus disease or normal result. We excluded exams of postoperative care of paranasal sinuses or skull base surgeries and those that has tumor masses in the region. Children below 13 years of age were also excluded, because the maxillary sinuses acquire their adult format at about this age, stabilizing its dimensions at about 18 years, and frontal and sphenoid sinuses become feasible for radiological identification at about 4 years of age6. In addition, below the age of 13 years, the occurrence of hypertrophied pharyngeal tonsil is higher, an important factor for the genesis of sinus disease in children that could influence the assessment of correlations of the anatomical variations of the nasal cavity with paranasal sinuses impairment.
CT scans were conducted using Philips Secura and Philips Tomoscan AV devices. The protocol included images at coronal section, perpendicular to the hard palate, frontal sinus (anterior limit) to the sphenoid sinus (posterior limit) with patients in supine position and neck hyperextension, using the correct head support for the position and sections of 3.0mm/3.00mm thick/increment with high resolution filter. The images collected at axial sections were parallel to the hard palate, from it (lower limit) to the frontal sinus (upper limit), with patients in supine and neutral head position, with correct head support, and sections of 3.0mm/3.0mm thick/increment. The images were made at 120/ 125 kV and 224/ 225 mAs. The tomographic windows used were 1500 - 2000/ 200 - 300 for bone parts and 300 - 400/ 30 - 40 (width/level) for soft parts.
The exams were analyzed concerning presence or absence of pathology in maxillary, ethmoid, frontal and sphenoid sinuses. In the present study, we adopted the classical concept of Som, who considered pathological any demonstrable area of mucosa, classified as a sinus disease 7.
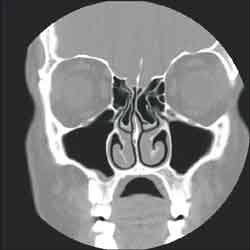
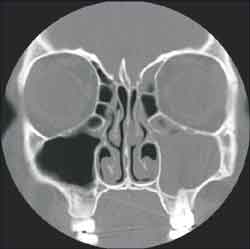
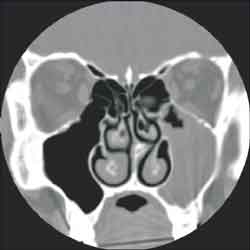
The studied anatomical structures were the middle concha, Haller cells, maxillary, ethmoid, frontal and sphenoid sinuses and the nasal septum (Figures 1, 2 and 3).
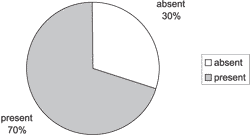
Graph 1. Distribution of sinus disease in paranasal sinuses CT scans.
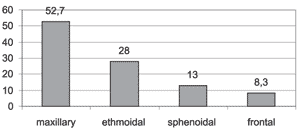
Graph 2. Distribution of most affected paranasal sinuses.
Graph 3. Distribution of anatomical abnormalities.
The middle concha was initially assessed and then classified as bullous when there was any pneumatization. Later, we studied the distribution of middle concha bullosa and the grades of sinus pathology.
We also checked presence or absence of Haller cells and their distribution in different grades of sinus disease. These cells are defined as pneumatization of the inferior-medial orbital rim, delimiting the upper-lateral limit of the ethmoid infundibulum, instead of the ethmoid bulla, as habitually found.
We also recorded the presence or absence of nasal septum deviation in the region of the middle meatus and its distribution in the different grades of sinus disease, disregarding deviations in any other region of the nasal cavity.
For the study of the correlation between the analyzed structures (middle concha bullosa, Haller cells and nasal septum deviation) and the grades of sinus disease, we used chi-square test, with significance level of 5%.
Figure 1 . Bilateral bullous middle turbinate (CT coronal section)
Figure 2. Bilateral Haller cel ( left obliterated) and left maxilo-etmoidal sinusopathy (CT coronal section)
Figure 3. Deviated septum to the left and left maxillo-etmoidal sinusopathy (CT coronal section)
Results
The sample consisted of 85 (56.7%) female subjects and 65 (43.3%) male subjects. The age ranged from 13 to 98 years (mean age of 38.2 years with standard deviation of 17.5 years).
In Graph 1, we can see sinus disease in 70% of the patients. The distribution of the most affected paranasal sinuses is shown in Graph 2. The analysis of CT scans (Graph 3) revealed that middle concha bullosa is the most frequently found anatomical abnormality, followed by septum deviation on the middle meatus and Haller cell.
We observed that there was no significant correlation between presence of Haller cell and maxillary, ethmoid and frontal sinus pathology (Tables 1, 2 and 3). In Tables 4, 5 and 6, we did not observe significant relation between presence of middle concha bullosa and maxillary, ethmoid and frontal sinus disease. Tables 7, 8 and 9 did not show significant correlation between nasal septum deviation on the middle meatus and maxillary, ethmoid and frontal sinus disease.Table 1. Distribution of maxillary sinus disease and presence of ipsilateral Haller cells.
Test: Chi-square
Observed Value: 0.00
Critical Value: 3,84
Table 2. Distribution of ethmoid sinus disease and presence of ipsilateral Haller cells.
Test: Chi-square
Observed Value: 0.09
Critical Value: 3.84
Table 3. Distribution of frontal sinus disease and presence of ipsilateral Haller cells.
Test: Chi-square
Observed Value: 0.70
Critical Value: 3.84
Table 4. Distribution of maxillary sinus disease and presence of ipsilateral middle concha bullosa.
Test: Chi-square
Observed Value: 1.61
Critical Value: 3.84
Table 5. Distribution of ethmoid sinus disease and presence of ipsilateral middle concha bullosa.
Test: Chi-square
Observed Value: 2.25
Critical Value: 3.84
Table 6. Distribution of frontal sinus disease and presence of ipsilateral middle concha bullosa.
Test: Chi-square
Observed Value: 1.58
Critical Value: 3.84
Table 7. Distribution of maxillary sinus disease and presence of ipsilateral septum deviation.
Test: Chi-square
Observed Value: 0.00
Critical Value: 3.84
Table 8. Distribution of ethmoid sinus disease and presence of ipsilateral septum deviation.
Test: Chi-square
Observed Value: 0,08
Critical Value: 3,84
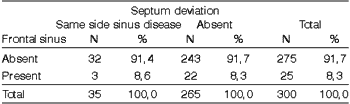
Table 9. Distribution of frontal sinus disease and presence of ipsilateral septum deviation.
Test: Chi-square
Observed Value: 0,00
Critical Value: 3,84
Discussion
Variations in agger nasi cells, middle conchae, uncinate process, as well as the presence of Haller cell or nasal septum deviation are mentioned as possible etiological factors for sinusal disease and headache8. Other factors that can be involved in the etiology of sinusitis include the contact of the mucosa which can take to loss of ciliar motility and interruption of transport, causing stasis of secretions and favoring bacterial infection3, 8.
Anatomical variations can cause additional stenosis of the lateral nasal wall, which can prevent the drainage and ventilation of the sinuses or cause obstruction. The variations can be asymptomatic and only cause a block during viral or bacterial infection, owing to mucosa edema9.
The studies that correlated anatomical variations to the occurrence of sinusitis are controversial. Some authors state that there is no association between anatomical variations and sinusitis10. Many of these studies were conducted only in populations of symptomatic patients.
Other authors compared symptomatic patients (or sinusal) and asymptomatic patients (or non-sinusal). The patients were considered asymptomatic when referred to the CT scan for other reasons, normally to conduct CT scan of the orbits11, 12. In this study design, there is a limitation upon the comparison of paranasal sinuses and orbit CT scans. Parameters for paranasal sinuses CT scans are very well defined, such as kilovoltage, milliamperage, duration of exposure, algorithm, plan, thickness and section spacing13. For orbit CT scan, all technical parameters are different, except for the section plan. Moreover, various studies have demonstrated an occurrence of mucous thickness in asymptomatic subjects from the nasal-sinusal perspective14, 15, 16. Therefore, we conclude that it is incorrect to use such patients as the control group.
In our opinion, the ideal selection of asymptomatic patients should be from healthy subjects, whose nasal-sinusal abnormalities are detected in clinical ENT examination and then are submitted to paranasal sinuses CT scan protocol defined by the literature. Therefore, it would be possible to know the real incidence of mucous thickness and the anatomical variations of the normal population. However, the exposure of healthy people to ionizing radiation raises ethical issues. For this reason, we decided to carry out the study with symptomatic patients, analyzing the intrinsic differences of the group.
In our study, we detected sinus disease in 70% of the patients (Graph 1), confirming the expectations based on the studied literature. Bolger et al.14 noticed that mucosal disease of the paranasal sinuses was found in 92.2% of the sinusal patients. Lloyd et al.17 observed it in 88% of the patients, Calhoun et al.18 in 62%, Tonai, Baba12 in 93%, Stackpole, Edestein19 in 58.4% and Kayalioglu et al.11 in 87.77%.
The most affected paranasal sinuses in decreasing order of frequency (graph 2) were: maxillary (52.7%), ethmoid (18.0%), sphenoid (13.0%) and frontal (8.3%). Our findings concerning the order of frequency were similar to those by Calhoun et al.18 and Stackpole, Edelstein19 The findings by Lloyd et al.17 were different only in the last two affected sinuses, since they found higher incidence of frontal than sphenoid sinuses. Zinreich et al.1 and Bolger et al.14 found in their studies the ethmoid sinus as the most affected one, followed by the maxillary sinus.
Zinreich et al.1 found mucous thickness in 72% of the CT scans of ethmoid sinus, 65% in maxillary sinus, 34% in frontal sinus, 40% in posterior ethmoid sinus and 29% in sphenoid sinus.
Bolger et al.14 noticed mucosa affection in paranasal sinuses of 92.2% of the sinusal patients. The anterior ethmoid region was the most affected one (84.3%), followed by maxillary (77.7%), frontal (36.6%), posterior ethmoid (38.6%), and sphenoid (25.4%).
Calhoun et al.18 observed sinusal abnormalities in 62% of the patients, being 43% in the maxillary sinus, 34% in anterior ethmoid cells, 21% in posterior ethmoid cells, 19% in sphenoid and 13% in frontal sinuses.
Loyd et al. 18 found 88% of patients with sinuses opacity: in 83% the maxillary antrum was affected, the ethmoid in 70% (63% anterior and 57% posterior), frontal sinus in 60% and sphenoid sinus in 49%.
Stackpole, Edelstein19 observed evidence of sinusitis mucosa in 58.4% of the patients. Maxillary sinusitis was observed in 47.4% of all patients, ethmoidal sinusitis in 35.7%, and frontal or sphenoidal sinusitis in 19.5%.
The incidence of Haller cells found in the present study was 9.3% (Graph 3). The literature data are very diffuse, raging from 3.65% to 45.9% 10, 11, 12, 14, 17,19, 20, 21, 22, 23, 24. Since all these authors adopted the same definition for Haller cells, this variation can correspond to intrinsic differences of the studied populations or differences in the sensitivity of detection of the methods employed. However, in some studies, the incidence of Haller cells was very high, which make us wonder whether the authors included hyperpneumatized ethmoidal bulla in the sample, owing to misinterpretation of the exam.
Haller cell is an expansion of the ethmoid cells and owing to its location it can potentially obstruct the ostium of the maxillary sinus and the ethmoidal infundibulum causing rhinosinusitis. Tables 1, 2 and 3 show that there is no statistically significant difference between presence of Haller cell and maxillary, ethmoid and frontal sinus affection.
Earwaker21 found 20% of Haller cells and suggested that such anatomical variation can be a significant etiological factor in recurrent maxillary sinusitis.
Stackpole, Edelstein19, upon analyzing 154 patients with sinusal problems observed an incidence of 34.4% of Haller cells. The cells were classified as small, middle and large in sizes. A statistically significant increase in maxillary sinus mucosa affection was observed in patients with middle and large sized Haller cells (45.8%) if compared to small cells (28.9%).
Most of the authors did not find a correlation between Haller cells and sinusal disease12, 14, 17. Regardless, we should carefully evaluated each patient and observe the dimensions of Haller cells, the presence of mucosa disease inside it and mucous contact.
The diagnosis of middle concha bullosa can be well made radiologically, since it is easily visualized by CT scan1. The incidence of middle concha bullosa in the present study was 33.3% (Graph 3). This distribution is in accordance with the literature which frequently refers to incidences that range from 21 to 55%1, 10, 11, 1, 14, 17, 18, 21, 22, 25, 26.
The difference in the incidence of middle concha bullosa observed in the literature can be the result of factors such as intrinsic differences of the studied populations, diagnostic criteria and sensitivity of the detection methods. Initial studies, by means of anatomical dissections, indicated low incidence of middle concha bullosa, between 8% and 20%. As a result of the advance in imaging tests, specially computed tomography, there has been improvement and increase in diagnosis of middle concha bullosa.
The definitions of middle concha bullosa vary. Some authors only consider it when there is extensive pneumatization, with increased volume7; others define middle concha bullosa only if it is bulbar27. The recent literature, however, defines as middle concha bullosa the presence of any degree of pneumatization subdividing it into lamellar, bulbar and extensive.
Finally, there are references to racial differences, such as less incidence of middle concha bullosa in Japanese people. The same study also described that whenever present, the grade of pneumatization is small12.
Tables 4, 5 and 6 show distribution of middle concha bullosa and the presence of paranasal sinus pathology and the absence of statistically significant difference between the variables.
Yousem et al.26 found an incidence of 33% of patients with middle concha bullosa. They observed a low coincidence between middle concha bullosa and maxillary and ethmoid sinusitis (23-24%), being that the size of the middle concha bullosa was more important than its presence.
Lloyd et al.17 found an incidence of 24% of middle concha bullosa and did not observe correlation between it and paranasal sinuses opacity. Scribano et al.24 did not observe correlation between middle concha bullosa and sinusal disease either.
Calhoun et al.18 observed that concha bullosa was more common in patients with sinusal disease (29.0%) than in non-sinusal patients (15.9%). They noticed that the presence of middle concha bullosa was associated with anterior ethmoid affection, but not with ostiomeatal complex disease.
Nadas et al. 25 found middle concha bullosa in 53% of the patients assessed with paranasal sinuses CT scan and observed that middle concha bullosa was not a significant factor in the genesis of abnormalities of the paranasal sinuses or ostiomeatal complex.
Tonai, Baba12 found 28.1% of middle concha bullosa in the sinusal group and 27.8% in the non-sinusal group. They reported that pneumatization of middle concha did not seem to be a very important factor for recurrent sinusitis, since such structure is rarely developed up to the point of obstructing the middle meatus.
In the present study, we found nasal septum deviation in 23.3% of the patients (Graph 3). We did not find statistically significant difference between presence of nasal septum and maxillary, ethmoid and frontal sinuses affections (Tables 7, 8 and 9). Blaungrund28 reported incidence of nasal septum deviation in 20%. Earwaker21 found septum deviations in 44% of the cases. Yousem et al.26, upon analyzing 100 paranasal sinuses CT scans, observed that patients with opacification of paranasal sinuses Presented more marked septum deviation; however, the presence of isolated septum deviation did not increase incidence of sinus pathology. Kayalioglu et al.11 observed nasal septum deviation in 22.22% of the patients with sinusal symptoms and in 12% of the patients without sinusal symptoms.
Calhoun et al.18 observed that septum deviation was more common in sinusal patients (40.0%) than in non-sinusal patients (19.5%). The presence of septal deviation was significantly associated with ostiomeatal complex affection, anterior ethmoid disease and posterior ethmoid disease.
Elahi et al.29 observed correlation between ostiomeatal complex disease and septum deviation. They also detected a pattern in increased angles of the septal deviation and severity of the sinusal disease.
The differences in values detected for the incidence of nasal septum deviation can be explained by the assessment criteria of this anatomical variation concerning its grade and location. In the present study, we considered only nasal septum deviations located in the middle meatus.
Conclusions
In the present study, after analyzing 150 CT scans of paranasal sinuses, we concluded that:
1. In patients with clinical symptoms of sinusitis, submitted to paranasal sinuses CT scan, the most frequently affected sinuses in decreasing order are: maxillary, ethmoid, sphenoid and frontal.
2. The presence of middle concha bullosa, Haller cells or nasal septum deviation in the middle meatus does not have statistically significant difference related to maxillary, ethmoid and frontal sinus affection. It is likely that intrinsic variations of the nasal and paranasal mucosa can influence more significantly the variations of the ostiomeatal complex.
References
1. Zinreich SJ, Mattox DE, Kennedy DW, Chiisholm HL, Diffley DM, Rosenbaum AE. Concha bullosa: CT evaluation. J Comput Assist Tomogr 1988;12:778-84.
2. Kennedy DW, Zinreich SJ, Rosenbaum AE, Johns ME. Functional endoscopic sinus surgery: theory and diagnostic evaluation. Arch Otolaryngol 1985;111:576-82.
3. Messerklinger W. On the drainage of the normal frontal sinus of man. Acta Otolaryngol 1967;63:176-81.
4. Stammberger H. Endoscopic endonasal surgery - concepts in treatment of recurring rhinosinusitis. Part I. Anatomic and pathophysiologic considerations. Otolaryngol Head Neck Surg 1986;94(2):143-47.
5. Zinreich SJ, Kennedy DW, Rosenbaum AE, Gayler BW, Kumar AJ, Stammberger H. Paranasal sinuses: CT imaging requirements for endoscopic surgery. Radiology 1987;163:769-75.
6. Navarro JAC. Cavidade do nariz e seios paranasais. Bauru: All Dent; 1997. 160 p.
7. Som PM. CT of the paranasal sinuses. Neuroradiology 1985;27:189-201.
8. Stammberger H, Wolf G. Headaches and sinus disease: the endoscopic approach. Ann Otol Rhinol Laryngol 1988;97(134):3-23.
9. Kopp W, Stammberger H, Fotter R. Special radiologic imaging of paranasal sinuses. Eur J Radiol 1988;8:153-6.
10. Ünlü HH, Akyar S, Çaylan R, Nalça Y. Concha bullosa. J Otolaryngol 1994;23(1):23-7.
11. Kayalioglu G, Oyar O, Govsa F. Nasal cavity and paranasal sinus bony variations: a computed tomographic study. Rhinology 2000;38(3):108-13.
12. Tonai A, Baba S. Anatomic variations of the bone in sinonasal CT. Acta Otolaryngol Suppl (Stockh) 1996;525:9-13.
13. Babbel R, Harnsberger HR, Nelson B, Solkens J, Hunt S. Optimization of techniques in screening CT of the sinuses. AJR 1991;157:1093-98.
14. Bolger WE, Butzin CA, Parsons DS. Paranasal sinus bony anatomic variations and mucosal abnormalities: CT analysis for endoscopic sinus surgery. Laryngoscope 1991;101:56-64.
15. Diament MJ, Senac MO Jr, Gilsanz V, Baker S, Gillespie T, Larsson S. Prevalence of incidental paranasal sinuses opacification in pediatric patients: a CT study. J Comput Assist Tomogr 1987;11(3):426-31.
16. Havas TE, Motbey JA, Gullane PJ. Prevalence of incidental abnormalities on computed tomographic scans of the paranasal sinuses. Arch Otolaryngol Head Neck Surg 1988;114:856-9.
17. Lloyd GAS, Lund VJ, Scadding GK. CT of the paranasal sinuses and functional endoscopic surgery: a critical analysis of 100 symptomatic patients. J Laryngol Otol 1991;105:181-5.
18. Calhoun KH, Waggenspack GA, Simpson CB, Hokanson JA, Bailey BJ. CT evaluation of the paranasal sinuses in symptomatic and asymptomatic populations. Otolaryngol Head Neck Surg 1991;104(4):480-3.
19. Stackpole AS, Edelstein DR. The anatomic relevance of the Haller cell in sinusitis. Am J Rhinol 1997;11(3):219-23.
20. Danese M, Duvoisin B, Agrifoglio A, Cherpillod J, Krayenbuhl M. Influence des variantes anatomiques nasosinusales sur les sinusites récidivantes, persistantes ou chroniques. Évaluation TDM chez 112 patients. J Radiol 1997;78(9):651-7.
21. Earwaker J. Anatomic variants in sinonasal CT. RadioGraphics 1993;13:381-415.
22. Meloni F, Rovasio S, Stomeo F, Teatini GP. Anatomic variations of surgical importance in ethmoid labyrinth and sphenoid sinus. A study of radiological anatomy. Surg Radiol Anat 1992;14:65-70.
23. Milczuk HA, Dalley RW, Wessbacher FW, Richardson MA. Nasal and paranasal sinus anomalies in children with chronic sinusitis. Laryngoscope 1993;103(3):247-52.
24. Scribano E, Ascenti G, Loria G, Cascio F, Gaeta M. The role of the ostiomeatal unit anatomic variations in inflammatory disease of the maxillary sinuses. Eur J Radiol 1997;24(3):172-4.
25. Nadas S, Duvoisin B, Landry M, Schnyder P. Concha bullosa: frequency and appearances on CT and correlations with sinus disease in 308 patients with chronic sinusitis. Neuroradiology 1995;37(3):234-7.
26. Yousem DM, Kennedy DW, Rosenberg S. Ostiomeatal complex risk factors for sinusitis: CT evaluation. J Otolaryngol 1991;20:419-24.
27. Ferrie JC, Azais O, Vandermarcq P, Klossek JM, Drouineau J, Gasquet C. Exploration tomodensitométrique de l'ethmoïde et du méat moyen. II. Radio-anatomie (incidence axiale) et variations morphologiques. J Radiol 1991;72(10):477-87.
28. Blaugrund SM. The nasal septum and concha bullosa. Otolaryngol Clin North Am 1989;22:291-306.
29. Elahi MM, Frenkiel S, Fageeh N. Paraseptal structural changes and chronic sinus disease in relation to the deviated septum. J Otolaryngol 1997;26(4):236-40.
30. Basic N, Basic V, Jukic T, Basic M, Jelic M, Hat J. Computed tomographic imaging to determine the frequency of anatomical variations in pneumatization of the ethmoid bone. Eur Arch Otorhinolaryngol 1999;256(2):69-71.
1 Post-graduate studies under course, Department of Otorhinolaryngology and Human Communication Disorders, Federal University of São Paulo - Escola Paulista de Medicina.
2 Joint Professor, Discipline of Otorhinolaryngology, Department of Otorhinolaryngology and Human Communication Disorders, Federal University of São Paulo - Escola Paulista de Medicina.
3 Joint Professor, Department of Imaging Diagnosis, Federal University of São Paulo - Escola Paulista de Medicina.
Study conducted at Federal University of São Paulo - Escola Paulista de Medicina
Financial Support: CNPq
Address correspondence to: Marcio Massao Kinsui - Rua Primitiva Vianco, 244, sala 1010 - 06016-901 Osasco SP - Tel: (55 11) 3654-3610 - Fax: (55 11) 3681-9111 - E-mail: marciokinsui@hotmail.com
Article submitted on July 30, 2002. Article accepted on August 15, 2002