Year: 2014 Vol. 80 Ed. 2 - (10º)
Artigo Original
Pages: 138 to 145
Amplitude and speed of masticatory movements in total laryngectomy patients
Author(s): Paulo Fernandes Pinheiro Junior1; Lucas Carvalho Aragão Albuquerque2; Cicilia Lais de Lima Silva3; Natália Freira da Silva3; Daniele Andrade da Cunha3; Hilton Justino da Silva3
DOI: 10.5935/1808-8694.20140029
Keywords: Electrophysiology; Rehabilitation; Aged
Abstract:
INTRODUTION: Laryngectomy is a surgical procedure that aims to remove the hyoid bone and the larynx and its muscles; it is inferred that a destabilization of the hyoid-mandibular axis will occur, consequently changing chewing.
OBJECTIVE: To characterize the amplitude and speed of chewing in laryngectomies and to compare them with two groups of non-laryngectomized individuals differentiated by age.
METHOD: 72 volunteers were divided into three groups: (A) 32 volunteers, mean age 22.3 years, 17 females; (B) 20 volunteers, mean age 53.2 years, 10 females, and (C) 20 volunteers who underwent total laryngectomy, mean age 61.5 years, one female. Electrognathography, a method that tracks and measures millimeter jaw movements, was used for evaluation.
RESULTS: Significant diferences were observed between groups A and C in masticatory amplitude and speed.
CONLCUSION: Age, and adaptive and compensatory changes appear to explain chewing better than factors related to total laryngectomy.
![]()
INTRODUCTION
Total laryngectomy is a method for the surgical treatment of laryngeal cancer. The procedure is characterized by a complete larynx removal, from the base of the tongue to the trachea, including the hyoid bone and the pre-epiglottic space,1-3 implying the mutilation of various systems and consequent alteration of their functional characteristics, such as in the stomatognathic system.4,5
The mutilations between the supra and infra-hyoidal regions that connect the jaw, hyoid bone, and external and clavicle2,6 directly interfere with the function of the stomatognathic system, leading the patient to develop compensatory neuromuscular parafunctions with the remaining muscular structures.7,8 These parafunctions represent the intricate impact on mandibular biomechanics derived from damage to structures that belong or are related to the stomatognathic system.9-11 One way to analyze these biomechanics is the measurement of amplitude and velocity of jaw movements, using predictive variables such as kinematic changes. In this context, electrognathography (EGN), a method tracks movements using magnetoresistive sensors, can be an excellent way to obtain these data.12,13
The measurement of amplitude and speed of movements related to the mandibular biomechanics of total laryngectomy patients has not yet been reported in the literature. The importance of this work was the quantitative data survey, which can support, reconfigure, or assist multidisciplinary therapeutic programs targeted at this issue.
This study aimed to characterize the amplitude and speed of masticatory cycles on total laryngectomy patients evaluated by electrognathography and to compare these data with those of two other groups of non-laryngectomized individuals differentiated by age.
METHODS
This was a cross-sectional, observational, exploratory, case series study with comparison groups, conducted between February and July of 2012. It included patients treated with total laryngectomy and volunteers without cirugical interviwe on neck or head. In addition to these study sites.
The main criteria adopted were patients in the postoperative period restricted to total laryngectomy with neck dissection, in the absence of recent post-operative complications or difficulty in understanding simple instructions. The study population comprised 20 patients with mean age of 61.5 years and mean postoperative period of 3.65 years, one (5%) of whom was a female (group C).
The type of sample was defined by the characteristics of the group of non-laryngectomized subjects. For this reason, non-probabilistic sampling was adopted, by accessibility or convenience; it builds on the subject specificity and allows them to represent the universe, by being descriptive and exploratory.14
To determine the sample size of the comparison groups, identified as young-adult group (group A) and adult group (group B), a ratio of 1.6:1.0, 0.05 significance level, and 80.0% power of evidence were assumed. This estimated a sample of 31 individuals in group A and 19 in group B; adequate estimates for the group B retained more characteristics similar to the universe of laryngectomy patients.15
Groups A and B had as common inclusion criteria: individuals of both genders; dentate or partial or total edentulous, with or without a prosthetic or orthodontic mouthparts; not submitted to surgery in the head and neck regions; no complaints of dysphagia; not subjected to any physical therapy and/or speech treatment; and no difficulty in understanding simple commands. Exclusion criteria for groups A and B were presence of neurological, neuromuscular, or neurodegenerative diseases, and clinical diagnosis of acute symptoms of temporal-mandibular disorders at the time of testing.
The differential inclusion criteria between groups A and B was age. Group A included young adults aged between 18 and 29 years; in group B, subjects were aged 40 or older.
72 individuals of both genders participated in the study, with the following distribution: group A comprised 32 volunteers, mean age equal to 22.3 years; group B comprised 20 volunteers, mean age 53.2 years; group C consisted of 20 patients who underwent total laryngectomy.
Phases of data collection
After reading and signing the informed consent, volunteers underwent history and specific physical exams.
For the electrognathographic examination, the volunteer was instructed to sit comfortably in a chair with the head erect and eyes directed forward.
An electrognathographer, model JT-3D® (BioRESEARSH), was used; the collected data was analyzed using BioPak System.
In the collection of electrognathography parameters, a small magnet was originally fixed to the labial surface of the lower incisors corresponding to the midline level; the head support was symmetrically regulated.
To evaluate the masticatory cycle, a volunteer was asked to chew 15 g of bread for 20 seconds. The jaw function and the consequent movement of the magnetic sensor were captured by electrognathographer, and transmitted and recorded into a computer, enabling the visualization and analysis of all intra-border mandibular motion graphics.
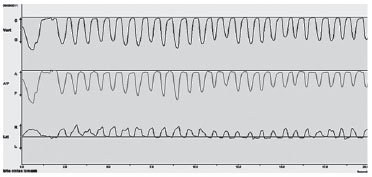
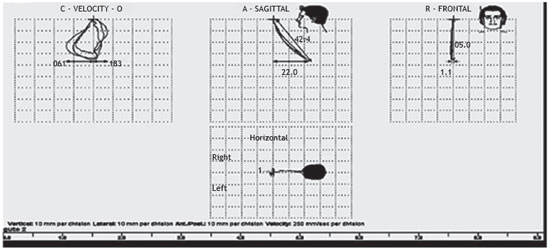
The display sweep (Fig. 1) analyzed the following variables: a) total number of cycles, b) number of lateral cycles to the right, c) number of lateral cycles to the left, d) number of protrusions, and e) number of retrusions. In the XY display (Fig. 2) the maximum values of the following variables were measured: a) opening amplitude in the frontal plane, b) right lateral movement in the horizontal plane, c) left lateral movement in the horizontal plane, d) protrusion in the sagittal plane, e) retrusion in the sagittal plane, f) maximum opening speed, and g) maximum closing speed.
Figure 1 Vert, total count of the number of cycles. Lat, number of masticatory cycles to the right and left, and A/P, number of protrusions and retrusions.
Figure 2 Velocity graph presents the maximum opening (the O side of the graph) and closing (C side of the graph) speeds. Sagittal graph presents the maximum protrusion (A side of the graph) and retraction (P side of the graph). Frontal graph presents the maximum opening amplitude. Horizontal graph presents the maximum right laterality (right side of graph) and maximum left laterality (left side of graph).
All measurements were summarized as mean, mean standard error, 95% confidence interval, median, and interquartile range. In the comparison among groups A, B and C, the ANOVA test was used for differences between means. In cases with no significant difference, Dunnett's post-hoc contrast was used to identify differences among groups, assuming group C as a parameter. In cases with no significance by ANOVA, Student's t-test was used for difference of means, also assuming group C as a parameter. In all tests, a 0.05 significance level was considered for rejecting the null hypothesis of equality of means between groups.
The study was approved by the local research ethics committee, under CAAE No. 0014.0.447.172-10. Resolution 196/96 of the Brazilian National Health Council and the Helsinki Declaration of 2008 were followed.
RESULTS
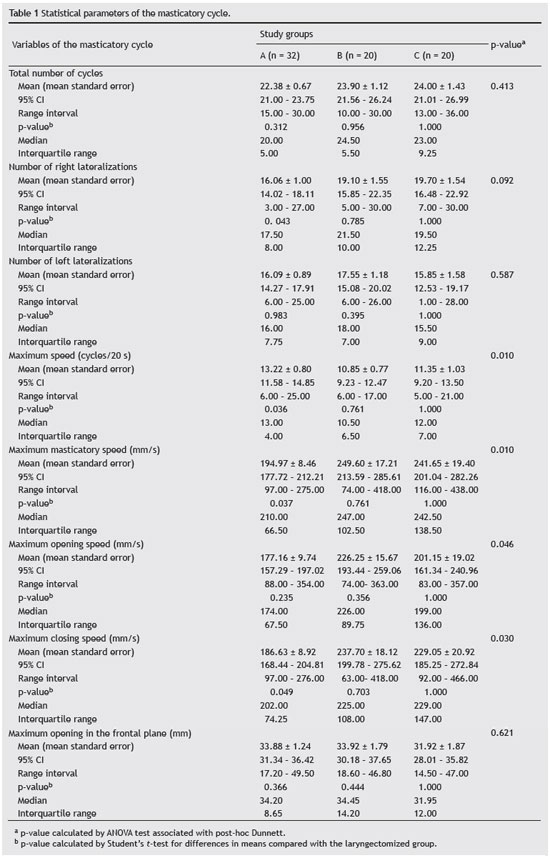
The number of masticatory cycles of laryngectomized patients was similar ingroups A and B, but differed in the frequency of lateralizations. The right lateralizations were more frequent than those to left, similar to that observed in adults in group B; however, these lateralizations reached higher frequency than that observed in group A (Table 1).
Hence the tendency to right lateralization resulted as a differentiation between the groups at the expense of major difference between group A and group C, which reached statistical significance. This difference, however, was absent in the left lateralization, for which there was no difference between groups, as well as in group C compared with groups A and B.
Regarding the speeds of masticatory cycles, a difference was observed among groups in the number of cycles per second and the maximum speed of mandibular displacement in both the opening and closing the mouth. Group C was significantly different from group A, with a lesser number of cycles per second and higher speed variation, which was proved by identifying the higher interquartile range.
Similarly, when analyzing the maximum displacement speed (mm/s), the significant difference observed among groups was due to the difference between groups C and A. Laryngectomized patients had greater displacement per time unit and greater variability in this displacement (Table 1). These differences were significant.
Regarding maximum mandibular displacement speed, it was observed that the average maximum opening speed reached significance only when comparing the means of three groups, but not when considering group C as the standard for comparison. Despite the lack of significance between groups A and B compared to group C, it is worth mentioning that the mean standard error of group C diverged more from group A than from group B. Regarding the means of maximum mandibular closing speed, the significance observed among groups was determined by the difference between group C and group A. Regarding the average of maximum opening evaluated in the frontal plane, there was no significant difference among groups (Table 1).
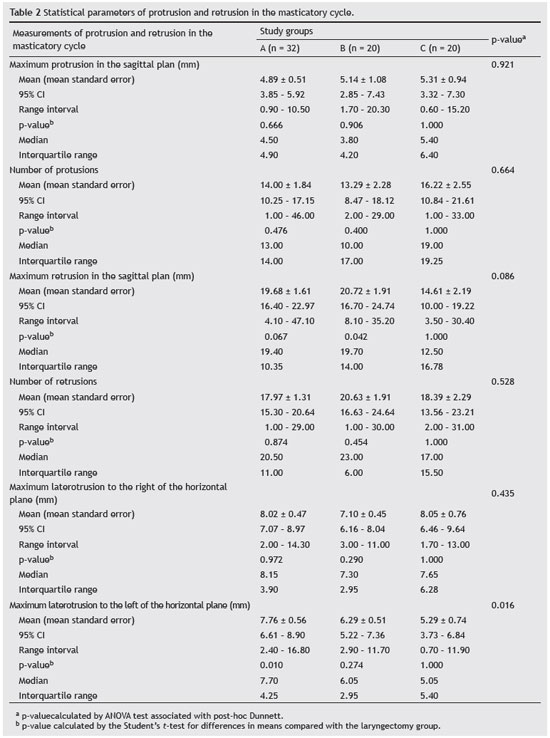
Table 2 shows the results of the statistical parameters related to the study of protrusions and retrusions found in the masticatory cycle of the three groups. There was no significant difference among groups and A and B in relation to group C, both in the number of protrusions and in the average of maximum protrusion assessed in the sagittal plane.
Regarding retrusions, the groups did not differ in frequency and variability. This difference was observed in the mean value of maximum retrusion; there was a statistical tendency of group C to have lower values than those in A and B, since the p-value was between 0.05 and 0.10. By comparing each group separately with the laryngectomized patients, a tendency for group C to present a mean lower maximum retrusion than that of group A and significantly lower mean than that of Group B was observed (Table 2).
The average maximum latero-retrusion, measured in the horizontal plane, had distinct behavior according to laterality. When shifted to the right, the values of group C were close to the measurements of groups A and B. However, in the shift to the left, these values were significantly lower in group C compared to the three groups, but with greater range of variation. From the comparison between group C, taken as standard, and the two other groups, it was observed that laryngectomized patients had mean maximum latero-retrusion to the left significantly lower than in group A (Table 2).
DISCUSSION
In recent years, several studies have been conducted on the possible relationships of supra- and infra-hyoid muscles and hyoid bone in chewing and in temporomandibular and cervical spine changes; so far, no agreement that could guide clinical approach has been reached.16 This difficulty evidences the great complexity of the issue and the multiplicity of factors that may be involved.11
The electrognathographic characterization of amplitude and speed of masticatory cycles in total laryngectomized patients, in turn, has demonstrated an even more complex mandibular biomechanics, which appeared to derive from the removal of the supra- and infra-hyoid muscles and hyoid bone, and to have been influenced by the aging process of muscles.
Research conducted to characterize the hyoid mechanism in the masticatory cycle has demonstrated the need for synergism between the involvement of the masseter, temporalis, and medium pterygoid muscles in mandible elevation, and the action of the supra-hyoid, anterior belly of digastric, milo-hyoid, genio-hyoid, lateral pterygoid, mimic, tongue, and infra-hyoid muscles as mandible depressors that depend on the hyoid bone position.17 The hyoid bone fixation by the infra-hyoid muscles enables the depressor muscles to promote mouth opening.18
Additionally, through electromyographic studies, there was also evidence of synergism between the extensor and flexor muscles of the head on the cervical spine and the activity of supra- and infra-hyoid muscles, and therefore the hyoid bone position, contributing to the stability of motion of the masticatory cycle.19
In this context, it is possible to assume that total laryngectomy, excising the supra- and infra-hyoid muscles, as well as the hyoid bone and tongue muscles, promotes new dynamics in the masticatory cycle, which appears to have been evidenced in the present study. Even considering that, in total laryngectomy patients, chewing suffers less quality of life impact than speech; the findings presented here are relevant in understanding these new kinematics.20
These new kinematics consisted of maintaining the number of masticatory cycles while changing the frequency and lateralization direction, which appeared to have been dictated mainly by the aging process, since the difference was observed when comparing the laryngectomy group with the younger group (group A); the difference was not observed in the group with similar age.
Compensatory and adaptive features of the stomatognathic system related to the age differential in the revelation of such data21,22 also appeared to explain the changes in speed expressed in number of masticatory cycles per second (cycles/20 s), whose qualitative value prevailed over the appearance of acquired by surgical action of total laryngectomy,20 since statistical significance was detected in group C, due to the smaller number of cycles per second and greater variation of velocity with respect to group A, expressed by a higher interquartile range (7.00 cycles/20 s in the group C versus 13.00 cycles/20 s in the group A).
However, the strongest suggestive evidence that aging might have contributed to masticatory adaptations were the means of maximum speed of mandibular displacement (generally in opening and closing), since group C resembled group B and differed significantly from group A in general displacement and at closing.
Variables such as maximal mouth opening in millimeters, maximum number of protrusions, and maximum protrusion in millimeters showed no significant differences. Although this finding has corroborated the evidence of Paula et al.20 that the masticatory characteristic is the least affected by total laryngectomy, it is plausible to assume that measurements of border movements may reveal statistically significant differences.23
Additionally, the trend of laryngectomy patients to show average maximum retrusion, maximum latero-retrusion evaluated in the horizontal plane ,and maximum left latero-retrusion smaller than those of the non-laryngectomy groups, showed for the first time the temporary or permanent disorder of stomathognatic functions, consequent to total laryngectomy and attributable to surgical manipulation in the supra- and infra-hyoid regions.24-26
CONCLUSION
The analysis of the variables suggested that changes of the masticatory cycle assessed by electrognathography evidence the existence of compensatory and adaptive traits in laryngectomized patients, resulting from the combined action of the age factor and changes possibly attributable to total laryngectomy.27,28
FUNDING
This study was supported by FACEPE (Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco).
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
1. Silva LSL, Pinto MH, Zago MMF. Assistência de enfermagem ao laringectomizado no período pós-operatório. Rev Bras Cancerol. 2002;48:213-21.
2. Genden EM, Ferlito A, Silver CE, Jacobson AS, Werner JA, Suarez C, et al. Evolution of the management of laryngeal cancer. Oral Oncol. 2007;43:431-9.
3. INCA, Câncer de Laringe. [cited 28 March 2010]. Available from: www.inca.gov.br.
4. Noronha MJR, Dias FL. Epidemiologia - etiopatogenia e fatores etiológicos do câncer da laringe - potencial para quimioprevenção. In: Noronha MJR, Dias FL, editors. Câncer da laringe: uma abordagem multidisciplinar. Rio de Janeiro: Livraria Editora Revinter; 1997. p. 4-7.
5. Hannickel S, Zago MMF, Bandeira CBS, Sawada NO. O comportamento dos laringectomizados frente a imagem corporal. Rev Bras Cancerol. 2002;48:333-9.
6. Noronha MJR. Tumores malignos da laringe. In: Brandão LG, Ferraz AR. Cirurgia de cabeça e pescoço. São Paulo: Editora Roca; 1989.
7. Santos J Jr. Oclusão clínica - atlas colorido. São Paulo: Santos; 1995.
8. Okeson JP. Tratamento das desordens temporomandibulares e oclusão. 4th edition. São Paulo: Artes Médicas; 1991.
9. Mannheimer JS, Rosenthal RM. Acute and chronic postural abnormalities as related to craniofacial pain and temporomandibular disorders. Dent Clin North Am. 1991;35:185-208.
10. Grimaldi N, Sarmento V, Provedel L, Almeida D, Cunha S. Conduta do cirurgião-dentista na prevenção e tratamento da osteorradionecrose: revisão de literatura. Rev Bras Cancerol. 2005;51:319-24.
11. Grade R, Caramês J, Pragosa A, Carvalhão J, Sousa S. Postura e disfunção temporo-mandibular: controvérsias actuais. Rev Port Estomatol Cir Maxilofac. 2008;49:111-7.
12. Paiva GD. Diagnóstico informatizado. In: Barros JJ, Rode SM, editors. Tratamento das disfunções craniomandibulares: ATM. São Paulo: Santos; 1995. p. 119-20.
13. Luz, JM. Medição de deslocamento através de sensores magnetoresistivos aplicada ao movimento mandibular. [Thesis]. Porto Alegre, Universidade Federal do Rio Grande do Sul; 2002.
14. Marotti J, Galhardo APM, Furuyama RJ, Pigozzo MN, Campos TN, Laganá DC. Amostragem em pesquisa clínica: tamanho da amostra. Rev Odontol Universidade São Paulo. 2008;20:186-94.
15. Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions. 3rd edition. Hoboken, NJ: Wiley-Interscience; 2003.
16. Tallgreen A, Lang B, Walker G, Ash M. Changes in jaw relations, hyoid position and posture in complete denture wearers. J Prosthet Dent. 1983;50:148-56.
17. Molina OF, Bielencki CO. Teste de provocação sobre a dor e fadiga: um estudo preliminar e implicações diagnósticas. J Bras Ortodon Ortop Facial. 1998;3:61-79.
18. Steenks MH, Wijer A. Disfunções da articulação temporomandibular do ponto de vista da fisioterapia e odontologia - diagnóstico e tratamento. São Paulo: Santos; 1996.
19. Forsberg CM, Hellsing E, Linder-Aaronson S. EMG activity in neck and mastigatory muscles in relation to extension and flexion of the head. Eur J Orthod. 1985;7:177.
20. Paula FC, Chaud RR. Avaliação de qualidade de vida em laringectomizados totais. Rev Bras Cir Cabeça Pescoço. 2009;38:177-82.
21. Ericksson PO, Häggman-Henrikson B, Nordh H, Zafar H. Co-ordinated mandibular and head - neck movementes during rhythmic jaw activities in man. J Dent Res. 2002;79:1378-84.
22. Bumann A, Lotzmann U. Disfunção temporomandibular: diagnóstico funcional e princípios terapêuticos. Porto Alegre: Artmed; 2002.
23. Takahashi M, Hideshima M, Park I, Tanigushi H, Ohyama T. Study of mandibular movements in mandibulectomy patients - border movements and functional movements during mastication, deglutition and speech. J Med Dent Sci. 1999;46:93-103.
24. Zago MMF, Stoppa MJR, Marinez EL. O significado cultural de ser laringectomizado. Rev Bras de Cancerol. 1989;44:239-45.
25. Wünsch V Filho. The epidemiology of laryngeal cancer in Brazil. São Paulo Med J. 2004;122:188-94.
26. Salomão CHD, Melo AS, Carvalho EC. Incertezas do paciente a ser submetido à cirurgia de laringectomia total. Rev Enferm UFPE. 2008;2:55-60.
27. Okeson JP, O'Donnell J. Standards for temporomandibular evaluation in the pediatric patient. J Pediatric Dentist. 1989;11:329-30.
28. Bodin C, Lodetti G, Marinone MG. Temporo-mandibular joint kinetics and chewing cycles in children. A 3-year follow-up. Int J Paediatr Dent. 2002;12:33-8.
1. Department of Pathology, Universidade Federal de Pernambuco (UFPE), Recife, PE, Brazil
2. Department of Neuropsychiatric, Universidade Federal de Pernambuco (UFPE), Recife, PE, Brazil
3. Department of Speech, Universidade Federal de Pernambuco (UFPE), Recife, PE, Brazil
Corresponding author.
L.C.A. Albuquerque
E-mail: fono_lucas@hotmail.com
Received 18 May 2013.
Accepted 12 August 2013.