Year: 2014 Vol. 80 Ed. 1 - (13º)
Artigo Original
Pages: 60 to 67
Influence of smoking isolated and associated to multifactorial aspects in vocal acoustic parameters
Author(s): Aline Gomes Lustosa Pinto1; Agrício Nubiato Crespo2; Lucia Figueiredo Mourão3
DOI: 10.5935/1808-8694.20140013
Keywords: Smoke; Voice; Acoustics
Abstract:
INTRODUCTION: Smoking affect voice quality in a long period of time, but other factors may compromise it, such as professional using of voice, habits, alcohol dependence and GERD. The aim was associate the influence of these factors on vocal parameters.
STUDY DESIGN: Contemporary cohort study with cross-sectional.
MATERIALS AND METHODS: Eighty adults of 35 to 60 years old had participated in this study, they had been divided into two groups, smokers (GF) and control (GC). There was application of questionnaire and voices were recorded. Praat software has been used for voice assessment and Man-Whitney, chi-square and logistic regression has been used for statistical analysis.
RESULTS: The GF had a higher incidence of alcohol dependence, coughing, throat clearing and professional voice using. Respecting to the acoustic parameters: noise-to-harmonic ratio (NHR), jitter and shimmer, the GF presented higher values. Relating these data to the questionnaire, it's noticed that female gender have influence over all acoustic parameters, GERD have influence over jitter and smoking can affect fundamental frequency, jitter, shimmer and NHR.
CONCLUSION: Smoking interferes in acoustics parameters isolated and associated with alcohol dependence, GERD, cough, throat clearing, gender and professional using of voice.
![]()
INTRODUCTION
The World Health Organization has declared that smoking is a public health problem, a habit that was popularized in the last century and persists today.1 Smoking causes chemical and psychological addiction, and can result in physiological changes, including vocal alterations.2
Prolonged exposure of the laryngeal mucosa to smoking compromises the mucoundulatory movement of the mucosa, changes voice quality, and causes a burning sensation, throat clearing, and the presence of secretions. Changes in voice quality were highlighted in the study by Sorensen and Horri3 and by Duarte et al.,4 who concluded that cigarette smoking modifies the voice, the histology of the region of the vocal folds, and favors the incidence of hyperplasia and metaplasia, contributing to the onset of cancer in this region.
Much is known about the isolated influence of smoking on voice quality changes; however, there have been few studies regarding this influence associated with other factors, such as age, gender, habits, professional voice use, alcohol abuse, upper airway infections, and gastroesophageal reflux (GER), factors that may enhance voice changes.
Alcohol consumption, whether or not associated with smoking, may contribute to the development of laryngeal cancer and reduce the life expectancy of its users.5-7 Regarding vocal habits, such as coughing and throat clearing, studies have found that the presence of these habits is common among smokers, and they are considered as risk factors for dysphonia.8,9 Another factor that can cause voice changes is the excessive vocal demand by professional use of the voice, which contributes to the incidence of vocal disorders.10,11
The aforementioned factors alone have an effect on voice quality; when associated with smoking, they may enhance vocal alterations, which are also observed when GER is considered.
GER causes vocal alterations, among them: hoarseness, laryngospasm, idiopathic subglottic stenosis, and other symptoms that also influence voice quality, such as chronic cough and phlegm.12-14 It is known that objective tests for the diagnosis of GER are necessary and are regularly used, but some articles suggest that pH monitoring, esophagogram, flexible laryngoscopy, and upper digestive endoscopy (UDE), used for this diagnosis, do not have overall accuracy when used alone. According to Ronkainen et al., 40% of subjects diagnosed with GER have no endoscopic abnormalities, suggesting that other mechanisms must be involved in the pathogenesis of the disease.15-19 In turn, Eckley et al.20 suggested that symptoms associated with GER should be considered, as they can favor and may aggravate the disease.
Studies have demonstrated that gastroesophageal reflux may be associated with salivary pH and volume of saliva, responsible for maintaining the homeostasis of the oral cavity, pharynx, and upper digestive tract. Thus, both interfere with the digestive process and maintain a correlation with reflux symptoms.21 By correlating smoking, saliva, and the presence of GER, the studies by Rourk et al.22 and Konturek et al.23 demonstrated that smoking modifies the concentration of the epidermal growth factor in the saliva. In turn, Eckley (2004) suggested that the deficiency of this factor contributes to GER disease (GERD).24
The voice is the subject of several studies because it is related to quality of life, social interaction, and is a working tool for many professionals, so it is necessary to identify the isolated influence of smoking on voice quality, as well as associated with other factors that can aggravate it, so that these factors can be considered from the point of view of treatment/rehabilitation. The aim of this study was to evaluate the influence of smoking as an isolated factor and as a factor associated with factors such as coughing and throat clearing habit, GER, alcoholism, and professional use of the voice on vocal acoustic parameters.
SAMPLE
The study was submitted to and approved by the Research Ethics Committee of the Faculdade de Ciências Médicas da Universidade Estadual de Campinas (CEP # 387/2010). A comparative clinical study was carried out for this purpose.
The sample consisted of 80 adult subjects aged 35 to 60 years from the city of Campinas, state of São Paulo, Brazil and surrounding region, of whom 40 were smokers (20 men and 20 women), and 40 were non-smokers (20 men and 20 women), comprising the smoker group (SG) and the control group (CG), respectively.
Adults aged between 35 and 60, who signed the informed consent, had normal hearing levels or mild to moderate hearing loss, no history of asthma or bronchitis, and who did not smoke or had smoked for less than 1 year were included in the CG. The SG included individuals that had smoked for over 20 years, with normal hearing or mild to moderate hearing loss, and no history of asthma or bronchitis.
Individuals that had smoked for less than 20 years, current non-smokers that had smoked for more than 1 year, and smokers or non-smokers with a medical diagnosis previous to the study of organic lesions in the laryngeal region were excluded from the study.
Data collection
For data collection, the sample subjects answered a structured questionnaire and were submitted to voice recording for acoustic analysis.
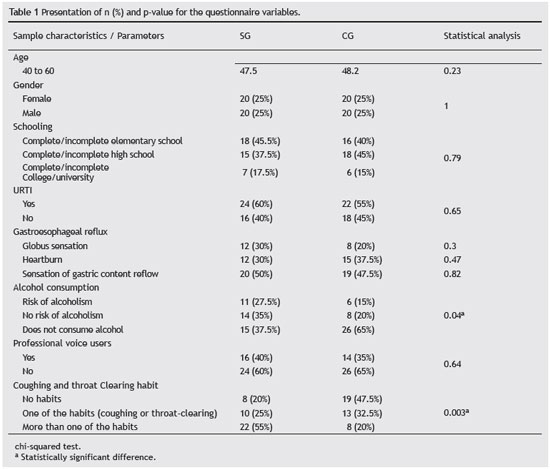
The structured questionnaire was applied in order to distribute the subjects between the groups, and it contained specific questions for the SG and CG to acquire information on demographic data such as level of education (elementary school, high school, or college/university), excluding subjects that had respiratory disorders such as asthma and bronchitis. Associated factors that could influence voice quality were also assessed, such as age, gender, presence of cough and throat clearing habit, professional use of the voice, upper respiratory tract infection, signs and symptoms of GER, general health status, and alcohol consumption, assessed by the CAGE scale (Table 1).25
The CAGE scale consists of a self-informative screening test validated for Brazil by Castells and Furllaneto27 and widely used in psychiatry. The scale consists of four questions, namely: Have you ever felt you should Cut down on your drinking? Have people Annoyed you by criticizing your drinking? Have you ever felt bad or Guilty about your drinking? Have you ever had a drink first thing in the morning (Eye opener) to steady your nerves or to get rid of a hangover? Two or more affirmative answers mean there is a risk of alcoholism. This evaluation tool was selected because it is used in most studies associating alcohol addiction and smoking.25-27
To know the signs and symptoms suggestive of GER, a questionnaire consisting of three questions based on the scale of typical symptoms for GER, created and validated by Velanovich was applied.28 Thus, according to the questionnaire, patients were considered to be at risk for the presence of GER when they answered affirmatively to just one question.28 The questions that characterized the symptoms suggestive of GER for the present study were: Do you feel a lump in your throat (frequency)? Do you have heartburn (frequency)? Do you have the sensation of food or liquids coming back from the stomach toward your mouth?
The voices were recorded in an environment with low level of noise for the vocal acoustic analysis. Subjects were asked to stand, maintaining a distance of 10 cm between the microphone and the mouth, and after a deep inspiration, they emitted the sound of the vowel /a/ in a sustained mode frequency and at usual intensities, without using their reserved air.
The analysis excluded the start and end of the sample, so that the attack and/or decrease of intensity of the emission ending did not interfere. The most stable portion of the emission with a mean time of 10 s was selected. The recording of emissions and their analysis were performed using the Praat software at a sampling rate of 44 kHz.
The recordings were performed using a digital system: DV4-2012 computer, HP Pavillion, AMD 300 M, a 15.6-inch monitor, pre-Mobile M-Audio sound card, and SM-58 CAD microphone.
At the acoustic analysis, the following parameters were observed: fundamental frequency, vocal range, noise-to-harmonic ratio (NHR), parameters of frequency disturbance, jitter (local, absolute local, and ppq5), and amplitude, shimmer (local, local dB, apq5, and apq11).
STATISTICAL ANALYSIS
Inferential analysis of the questionnaire data was performed through the chi-squared test to check for differences between groups regarding age, gender, level of schooling, and to describe other data in the questionnaire. Comparison of acoustic parameters, vocal range, NHR, jitter, shimmer, and fundamental frequency between the SG and CG was performed using the Mann-Whitney test. Simple linear regression was used to analyze the acoustic parameters and explain them through the factors: gender, GER, cough and throat clearing habit, professional use of the voice, smoking, and alcohol consumption. A total of 80 subjects were considered for the simple regression analysis, using the parameter smoking as a variable, instead of separating them between smoker and nonsmoker groups. All analyses considered a significance level of 0.05 or 5%.
RESULTS
According to the acoustic parameters found, there was a statistically significant difference for the disturbance measures: local absolute jitter (ms), local shimmer (dB), apq11 shimmer (%), and NHR. A trend in males (p = 0.06) was observed for the fundamental frequency (p = 0.06).
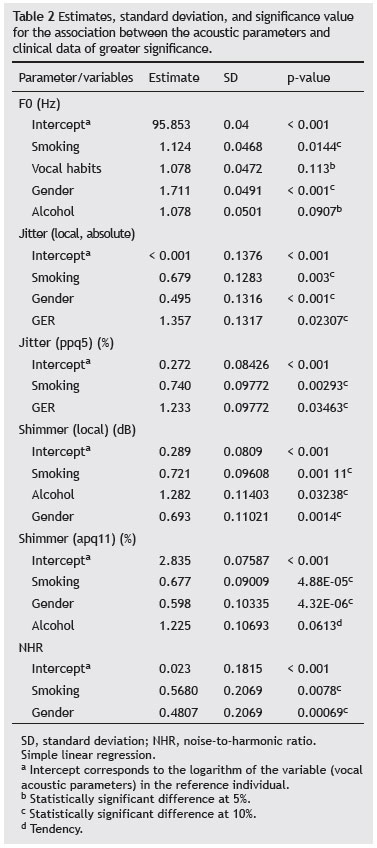
When considering the isolated study variables and the acoustic parameters through simple logistic regression, the association between them was established according to the statistically significant differences at levels of 5% and 10% for smoking and GER regarding the fundamental frequency; smoking, GER, and alcohol for local absolute jitter; smoking, GER, and gender for jitter ppq5; smoking, vocal habits, and gender for local shimmer dB; smoking, vocal habits, gender, and alcohol consumption for shimmer apq11; smoking and gender for NHR.
When considered together, it was observed that some variables persisted with a greater association with the acoustic parameters than others, such as smoking and gender for the fundamental frequency; smoking, GER, and gender for local absolute jitter; smoking and GER for jitter ppq5; smoking, gender, and alcohol consumption for local shimmer dB; smoking, gender, and alcohol consumption to shimmer apq11; smoking and gender for NHR.
In the present study, only variables with the highest level of significance regarding the acoustic parameters were considered (Table 2).
DISCUSSION
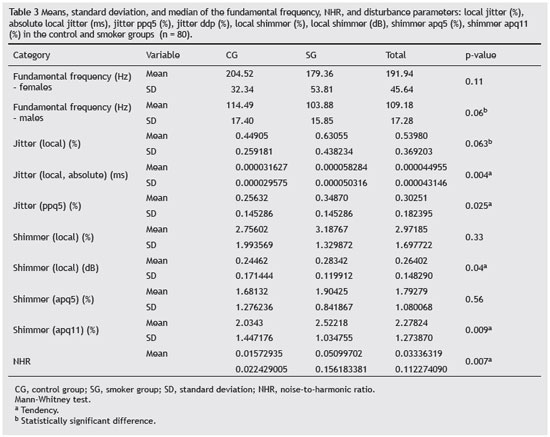
Smoking decreases fundamental frequency values in both genders (Table 3). For women, there are other factors that can contribute to reduce the fundamental frequency, such as menopause and Reinke's edema (RE), which has a higher incidence in women and is consistent with findings related to female subjects of this study.29,30
According to Queija et al. (2006), in a study of male smokers with a mean age of 53 years, who had smoked eight to 60 years, acoustic parameters such as the fundamental frequency and shimmer were slightly reduced. Similar results were found in the study by Figueiredo et al. (2003), which showed a tendency (p = 0.07) to decrease the fundamental frequency, similar to what was observed in the present study.31,32
Due to the low pitch found in male voices, the auditory perception of voice deepening in male smokers is less striking, despite the worsening in the fundamental frequency observed in the acoustic analysis, which may contribute to the maintenance of smoking.
Regarding the mean duration of smoking, the present study recruited subjects with a mean of 30 years, with a minimum smoking duration of 20 years. The standard deviation was 0.7, a low value, indicating that there was no wide variation in the duration of smoking among the subjects studied, making the results more robust. Other studies, such as those by Figueiredo et al.31 and by Queija et al.,32 recruited very young subjects, with reduced time of smoking, and subjects with a wide variability of duration of smoking, showing little control of this variable, which may have had an impact on results.
The fundamental frequency appears to be the acoustic parameter that shows the highest changes in value when the smoking factor is associated.The study by Queija et al.32 found more evident changes in the fundamental frequency when smokers and nonsmokers were compared at older ages, probably due to prolonged smoking, but it is a questionable result, when considering the age of the study subjects (35 to 81 years), due to the influence of senility on acoustic parameters - older subjects exhibit typical structural changes caused by aging, capable of modifying the acoustic parameters.33
The fact that the study sample consisted of adult subjects and that the duration of smoking was a controlled variable contributed to the fact that the results indicated no differences between smokers and nonsmokers, as observed in the study by Figueiredo et al.31 Alcohol addiction and the habit of coughing and throat clearing were also associated with the fundamental frequency; however, they were not significant 5% (Table 2).
The jitter represents the variation of the fundamental frequency when evaluating small irregularities in the glottal pulses, indicating the noise in the vocal signal. The measurement of its variations shown in this study, local absolute jitter (ms), the mean absolute difference between consecutive periods and jitter ppq5 (%), mean absolute difference between a period and its mean and its four nearest neighbors divided by the mean period were within normal limits for both groups (Table 3).32,33
According to the comparison made between the CG and SG, there was a statistically significant difference for the absolute jitter and jitter ppq5 (Table 3). Based on the literature, subjects who do not have laryngeal disorders have low values of these parameters; however, if these parameters were high, as in the case of smokers, the presence of diseases is considerable, suggesting the influence of smoking on these parameters.34
According to the analyzed parameters, there was a statistically significant difference at the 5% level for the association between smoking and jitter parameter, suggesting that the presence of this habit can be associated with changes in the values of this parameter, in agreement with the literature regarding the influence of smoking on jitter, due to vocal disorders related to smoking.
According to the literature, smoking is closely linked to laryngeal disorders, which can cause RE, leukoplasia, hyperplasia, and neoplasia, so that early surgical or speech therapy interventions accompanied by the cessation of smoking become necessary.5,33
GER and gender also showed statistically significant differences in vocal acoustic parameters (Table 2). GER can generate a series of laryngeal disorders that can cause hoarseness, roughness, and breathiness, among others. According to the study by Vashani et al.,35 it can be concluded that jitter is increased with reflux, highlighting the association identified in this study between its presence and changes in jitter values. The literature also highlights that, in the presence of speech therapy associated with drug treatment, this parameter tends to decrease.10,36 Notably, in the present study only the signs and symptoms suggestive of GER were considered, so that the subjects did not necessarily have a diagnosis of GER, which may have influenced the results of the present study.
For the gender variable, according to the literature, menopause begins around the age of 50 years, and yet its effects on the voice may emerge around the age of 40 years, during which there is a tendency of the superficial layer of the lamina propria to become edematous and thicker, which contribute to changing the vocal parameters, including increased jitter.30,36
In contrast, male individuals will have structural changes in the vocal tract only around the age of 60 years; thus, the primary factor that influences the male gender is the structural change caused by age.33 Therefore, the incidence of laryngeal alterations of anatomical origin is more common in females than in males, which may explain the changes in the jitter values in the presence of the gender factor (Table 2).
The shimmer represents the amplitude variation and is related to the decrease in the coefficient of contact of vocal folds. Measures of its variations evaluated in this study (local shimmer (dB), absolute mean (log10) of the difference between the amplitudes of consecutive periods, multiplied by 20; and shimmer apq11, mean absolute difference between the amplitude of a period and the mean of its amplitudes and its ten closest neighbors, divided by the overall mean amplitude) were outside the normal range for both groups (Table 3).32,33
When comparing the CG and SG, there was a statistically significant difference only for local shimmer (dB) and apq11. According to the literature high shimmer values are indicative of laryngeal disorders; Behlau33 observed that an increase in shimmer values occurs as a consequence of reduced glottal resistance, increased mass, and presence of noise and breathiness in the voice signal.
Table 2 shows that smoking and gender exhibited statistically significant differences, indicating that the presence of this habit is associated with changes in shimmer. This association was also established with gender, which is in agreement with the literature, and allows for the inference that there is an influence of menopause effects on shimmer.30,36 Regarding alcohol consumption, a tendency to increase the shimmer apq11 parameter and a statistically significant difference for local shimmer dB were observed.
The literature shows that cigarette smoking can increase the volume of the vocal fold epithelium, contribute to the onset of mass edema (such as RE), and cause the onset of laryngeal cancer. All these changes will impair voice quality, causing, among other characteristics, hoarseness and breathiness, which in turn are closely associated with shimmer.33
When associated with smoking, alcohol has a direct influence on the voice. The smoke-alcohol combination potentiates the morphological changes in the epithelium of the vocal folds, so that this epithelium is thicker in smokers and drinkers. For Hirabayashi et al. this fact reinforces the findings of the present study regarding the association of this variable and the shimmer parameter.37
The NHR assesses the presence of noise in the analyzed voice signal; higher NHR indicates greater noise in the voice signal and worse voice quality. This measure broadly assesses the analyzed noise in the signal, and is not specific to certain cycles, instead aiming to determine the overall noise in the voice signal.38
NHR values were increased in the SG when compared with the CG, with statistically significant difference between both groups (Table 3). As shown in Table 2, there was a statistically significant difference for the gender and smoking factors, which allowed for the inference that gender and the presence of these factors are associated with NHR.
The literature shows that cigarette smoking alters the voice quality, thus causing smokers to have roughness and fluid phonation, characterized by a lower degree of glottal coaptation, favoring the decreased amount of harmonics and increased presence of noise, as observed in the present study.37
Regarding the association between gender in NHR, according to the literature, the specific anatomical characteristics between genders and the menopause factor influence the other mentioned parameters and the NHR, as described in the present study.
Thus, it was observed that the gender factor maintains an association with the following acoustic parameters: jitter, shimmer, and NHR, whereas smoking was associated with the fundamental frequency, jitter, and shimmer, and GER was associated with jitter.
This study reinforces the need for further studies that assess the interrelationship between biological, social, and environmental factors, and habits such as throat clearing in voice production, as the vocal characteristics are influenced by different parameters, emphasizing the necessity of understanding the multifactorial etiology in the assessment and treatment of smokers and nonsmokers.
It is noteworthy that studies correlating voice quality with other factors such as those described in the present study are required for speech therapists and otolaryngologists to have a multifactorial approach in the treatment and prevention of factors that may worsen vocal quality.
CONCLUSION
The SG had a higher incidence of coughing, throat clearing and alcohol addiction when compared to the CG.
The values of the fundamental frequency in the SG were reduced when compared to the CG, but within normal limits for both groups; however, men have more deviant values from the expected mean for this parameter.
The parameters of the frequency disturbance (local absolute jitter and jitter ppq5) and intensity (shimmer local dB and shimmer apq11) were elevated in the SG.
The presence of smoking was associated with changes in the following parameters: fundamental frequency, jitter, shimmer, and NHR. The gender factor was also associated with changes in the parameters: jitter, shimmer, and NHR, which also suggests that smoking can increase the observed values. The presence of signs and symptoms suggestive of GERD and alcoholism leads to an association with jitter and shimmer, and suggests that the presence of these factors leads to the increase of the mentioned parameters.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
1. World Health Organization. Geneva: World Health Organization, 2007. World No Tobacco Day 2004 materials. [cited 01 Sep 2005] Available from: http://www.who.int/tobacco/resources/publications/wntd/2004/en. Accessed in 27/09/2011.
2. Rafferty MA, Fenton JE, Jones AS. The history, aetiology and epidemiology of laryngeal carcinoma. Clin Otolaryngol. 2001;26:442-6.
3. Sorensen D, Horii Y. Cigarrete smoking and voice fundamental frequency. J Commun Dis. 1982;15:135-44.
4. Duarte JL, de Faria FAC, Ceolin DS, Cestari TM, de Assis GF. Efeitos da inalação passiva da fumaça de cigarro sobre as pregas vocais de ratos. Rev Bras Otorrinolaringol. 2006;72:210-6
5. Hashibe M, Bofetta P, Zaridze D, Shangina O, Szeszenia-Dabrowska N, Mates D, et al. Contribution of tobacco and alcohol to the high rates of squamous cell carcinoma of the supraglottis and glottis in central Europe. Am J Epidemiol. 2007;165:814-20.
6. Shiga K, Tateda M, Katagiri K, Nakanome A, Ogawa T, Asada Y, et al. Distinct features of second primary malignancies in head and neck cancer patients in Japan. Tohoku J Exp Med. 2011,225:5-12.
7. Leon X, del Prado M, Orus C, Lopez M, Barcia J, Quer M. Influence of the persistence of tobacco and alcohol use in the appearance of second neoplasm in patients with head and neck cancer. A case control study. Cancer Causes Control. 2009;20:645-52.
8. Cielo CA, Finger LS, Niehues GR, Deuschle VP, Siqueira MA. Disfonia organofuncional e queixas de distúrbios alérgicos e/ou digestivo. Rev CEFAC. 2009;11:431-9.
9. Silverio KCA, Gonçalves CGO, Penteado RZ, Vieira TPG, Libardi A, Rossi D. Ações em saúde vocal: proposta de melhoria do perfil vocal de professores. Pró-Fono. 2008;20:177-82.
10. Fortes FSG, Imamura R, Tsuji DH, Sennes LU. Perfil dos profissionais da voz com queixas vocias atendidos em um centro terciário de saúde. Rev Bras Otorrinolaringol. 2007;73:27-31.
11. Williams NR. Occupational groups at risk of voice disorders. Occup Med (Lond). 2003;53:456-60.
12. Velanovich V. Gastroesophageal reflux disease and the airway essentials for the surgeon. World J Gastrointest Surg. 2009;1:8-10
13. Mahieu HF. Review article: the laryngological manifestations of reflux disease; why the scepticism? Aliment Pharmacol Ther. 2007;26:17-24.
14. Vaezi MF. Laryngeal manifestations of gastroesophageal reflux disease. Curr Gastroenterol Rep. 2008;10:271-7.
15. Abdo TRT, Yoshimura R, Mendonça RR, Frizzarini R, Santos RO, Rapoport PB. Doença do refluxo laringofaríngeo. Arq Med ABC. 2004;29:10-1.
16. Branski R, Bhattacharyya N, Shapiro J. The reliability of the assessment of endoscopic laryngeal findings associated with laryngopharyngeal reflux disease. Laryngoscope. 2002;112:1029-4.
17. Yliato R, Lindestad P, Ramel S. Symptoms, laryngeal findings, and 24-hours pH monitoring in patients with suspected gastroesophageal pharyngeal reflux. Laryngoscope. 2001;111:979-81.
18. Giacchi R, Sullivan D, Rothstein S. Compliance with anti-reflux therapy in patients with otolaryngological manifestations of gastroesophageal reflux disease. Laryngoscope. 2000;110:19-22.
19. Ronkainen JA, Aro P, Storskrubb T. Prevalence of esophagitis and endoscopy-negative reflux disease in a population. A report from the Kalixandra study. Abstr Dig Dis Week. 2002;S1357:A-269.
20. Eckley CA, Michelsohn N, Rizzo LV, Tadakoro CE, Costa HO. Salivary epidermal growth factor concentration in adults with reflux laryngitis. Otolaringol Head Neck Surg. 2004;131:401-6.
21. Costa HO, Neto OM, Eckley CA. Is there a relationship between the pH and volume of saliva and esophageal pH-metry results? Dysphagia. 2005;20:175-81.
22. Rourk RM, Namiot Z, Sarosiek J, Yu Z, McCallum RW. Impairment of salivary epidermal growth factor secretory response to esophageal mechanical and chemical stimulation in patients with reflux esophagitis. Am J Gastroenterol. 1994;89:237-44.
23. Konturek JW, Bielanski W, Konturek SJ, Bogdal J, Oleksy J. Distribution and release of epidermal growth factor in man. Gut. 1989;30:1194-200.
24. Costa HO, Eckley CA. Correlação do pH e volume salivares com sintomas laringofaríngeos. Rev Bras Otorrinolaringol, 2004;70:24-8.
25. Masur J, Moteiro MG. Validation of the CAGE alcoholism screening test in Brazilian psychiatric hospital setting. Braz J Med Biol Res. 1983;16:215-8.
26. Smart RG. Use of the CAGE scale in a population survey of drinking. J Stud Alcohol. 1991;52:593-6.
27. Castells MA, Furlanetto LM. Validade do questionário CAGE para rastrear pacientes com dependência ao álcool internados em enfermarias clínicas. Rev Bras Psiquiatr. 2005;27:54.
28. Velanovich V, Vallance ST, Gusz JR, Tapia FV, Harkabus MA. Quality of life scale for gastroesophageal reflux disease. J Am Col Surg. 1996;183:217-24.
29. Reinke FB. Untersuchungen über das menschlich Stimmband. Fortschritte der Medizin, München. 1985;13:469-78.
30. Machado MAMP, Aldrighi JM, Ferreira LP. Os sentidos atribuídos à voz por mulheres após a menopausa. Rev Saúde Pública. 2005;39:261-9.
31. Queija DS, Barros APB, Dedivitis RA, Corazza VR, Silva VFC, Nishimoto IN. Avaliação laringoestroboscópica, perceptiva e computadorizada da voz de tabagistas do gênero masculino. Rev Bras Cir Cabeça Pescoço. 2006;35:93-6.
32. Figueiredo DC, Souza PRF, Gonçalves MI, Biase NG. Análise perceptivo-auditiva computadorizada e laringológica da voz de adultos jovens fumantes e não fumantes. Rev Bras Otorrinolaringol. 2003;69:791-9
33. Behlau M. Voz: o livro do especialista. Vol I. Rio de Janeiro: Revinter; 2001.
34. Boersma P, Weenick D. Praat Manual. Amsterdam: University of Amsterdam, Phonetic Sciences Department; 2006. [cited 27 Sept 2011]. Available from: http://www.fon.hum.uva.nl/praat/.
35. Vashani K, Murugesh M, Hattangadi G, Gore G, Keer V, Ramesh VS, et al. Effectiveness of voice therapy in reflux-related voice disorders. Dis Esophagus. 2010;23:27-32.
36. Behlau MS, Pontes PAL. O desenvolvimento ontogenético da voz: do nascimento à senescência. In: Avaliação e tratamentos das disfonias. 1ª ed. São Paulo: Louise; 1995. p. 49-52.
37. Hirabayashi H, Hoshii K, Uno K, Ohgaki H, Nakasone Y, Fujisawa T, et al. Laryngeal epithelial changes on effects of smoking and drinking. Auris Nasus Laynx. 1999;17:105-14.
38. Teixeira VL. Avaliação da configuração laríngea em sujeitos portadores de Edema de Reinke. [Monograph] São Paulo: Centro de Saúde da Voz; 1999.
1. Speech Therapist, Hospital Vera Cruz, Campinas, SP, Brazil
2. Department of Otorhinolaryngology and Ophtalmology, Faculdade de Ciências Médicas, Universidade Estadual de Campinas (UNICAMP), Campinas, SP, Brazil
3. Speech Therapist, Faculdade de Ciências Médicas, Universidade Estadual de Campinas (UNICAMP), Campinas, SP, Brazil
Corresponding author.
A.G.L. Pinto
E-mail: lineglp@gmail.com
Received 15 December 2012.
Accepted 22 September 2013.