Year: 2014 Vol. 80 Ed. 1 - (6º)
Artigo Original
Pages: 18 to 23
Influence of estradiol administration on estrogen receptors of nasal mucosa: an experimental study on guinea pigs
Author(s): Henrique Olival Costa; Ney Penteado de Castro Neto; Lia Mara Rossi; Ieda Millas; Flavia Coelho; Leonardo da Silva
DOI: 10.5935/1808-8694.20140006
Keywords: Nasal mucosa; Estrogens; Immunochemistry
Abstract:
INTRODUCTION: Some clinical trials revealed a correlation between increased serum estrogen and nasal symptoms or inflammatory changes in nasal mucosa. Estrogen receptors tend to be controlled by a negative feedback, to avoid a deleterious stimulus over several body functions while in hyperestrogenic periods. This study proposes a hypothesis where mechanisms regulating expression of estradiol receptors in nasal mucosa are absent in some patients, and their concentration remains steady even in periods of high serum hormonal concentration, potentially leading to local estrogenic symptoms in nasal mucosa.
STUDY DESIGN: This was an experimental prospective study.
AIM: To determine whether estrogen levels induce the reduction of the number of estrogen receptors in the nasal mucosa.
METHODS: In the present study, 30 adult male guinea pigs were subjected to a biopsy of the middle nasal turbinate and received 0.5 mL of estradiol cypionate intraperitoneally for 30 consecutive days. Afterwards, samples from contralateral middle turbinate were obtained. Immunohistochemical analysis of estrogen receptors were performed pre- and post-treatment.
RESULTS: The post-treatment group showed reduction of receptor expression when compared to the pre-treatment group. (p = 5.2726-5).
CONCLUSION: A reduction in the expression of the nasal estrogen receptor was observed after 30 days of estradiol administration.
![]()
INTRODUCTION
Rhinitis may be classified into two groups: allergic and non-allergic. The non-allergic group includes hormonal rhinitis, in which inflammatory alterations in nasal mucosa are associated to elevated hormone levels, such as hyperestrogen states occurring during pregnancy, menstruation, or with use of hormonal contraception.1-3
Hormonal rhinitis has been the focus of clinical trials since the 19th century, when several authors published studies correlating hormonal fluctuations with nasal symptoms. Many authors4-7 carried out experimental studies disclosing histological and histochemical changes in nasal mucosa in response to changes in the concentration of serum estrogen.
Some clinical trials8-16 observed a correlation between increased serum estrogen and nasal symptoms or inflammatory changes in nasal mucosa on clinical and laboratory exams. Conversely, other studies, such as those by Mabry,17 by Ellegard,18,19 by Bende and Gredmark,20 and by Salaroli et al.21, failed to confirm this correlation.
Toppozada et al.22-24 observed histological and histochemical alterations in the human nasal mucosa of women who were pregnant or using contraceptive pills, compared with a control group. Caruso et al.12 and Nappi et al.25 noticed that the vaginal and nasal respiratory epithelium exhibited the same histologic aspects in the respective phases of the menstrual cycle and during menopause.
The first estrogen receptor (ER) to be described, alpha (ERα), was isolated by Elwood Jensen in 1958. In 1996, Enmark and Gustafsson26 discovered a second type of receptor, beta (ERβ). Theories proposed about the physiological functions of these two isoforms implicate selective action of estrogen in different tissues.
Thus, research into the two receptors subtypes has been conducted in a range of different organs and tissues. ERβ was found in the prostate, ovaries, testicles, uterus, hypophysis, bladder, lungs, salivary glands, oral mucosa, thymus, adrenals, olfactory tract, central nervous system, heart, kidneys, and in cells of the immunologic system.27 ERα was found at highest concentrations in the uterus, vagina, and breasts;28 however, few studies investigating ERs in nasal mucosa are available.
The mucosa of lower nasal turbinates in women with chronic rhinopathy was investigated by immunohistochemistry for ERs, which were dectected in the cytoplasm of glandular epithelium cells.29,30 Millas31 employed immunohistochemistry to study estrogen ERα and ERβ in the mucosa of lower nasal turbinates of normal subjects. All 11 cases studied (five women and six men) presented ERα and ERβ (predominantly the latter) in the cytoplasm of glandular epithelial cells of the lamina propria. When studying individuals with chronic rhinopathy, Shirasaki et al.32 observed ERα in the nuclei of mastocytes, and ERβ in nuclei of cells of the glandular epithelium of the lamina propria.
Controversy remains over the site and function of receptors for estrogen in nasal mucosa. Millas et al.33 assessed the influence of oral contraceptives on the distribution and density of ERs in the nasal mucosa of women, and observed that those using contraceptive pills had a lower number of receptors in the lamina propria.
The expression of receptors in cells is dynamic and regulated mainly by the concentration of their cognate ligands. Reports by Jensen and Gorski describing the regulation of ER concentration observed that estrogen treatment led to a decrease in cytosolic ERs, based on the reduction in specific binding to 17β-estradiol. Given that the receptors are the key determinants of the action of several hormones in target cells, and that evidence points to a possible estrogen activity in nasal mucosa, the mechanism of down-regulation may explain the fact that, in general, women do not present significant nasal effects when there is marked variation in their sexual hormones.
Inversely, a hypothesis may be proposed that mechanisms regulating receptors expression are absent and that their concentration remains steady even during high hormonal concentration, potentially leading to local estrogenic symptoms in nasal mucosa. This study aimed to determine whether an elevation in circulating estrogen levels influences the concentration of ERs in guinea pig nasal mucosa.
MATERIALS AND METHODS
A prospective study of nasal mucosa samples from 30 adult male guinea pigs weighting between 300 g and 400 g was performed.
The procedures were conducted in accordance with the regulations of the research ethics committee of the Unit for Experimental Surgery Technique and complied with Brazilian Federal Law No. 1153/95 as well as with the ethical principles for animal experiments established by the Brazilian College of Animal Experimentation (Colégio Brasileiro de Experimentação Animal - COBEA).
Procedures
The surgical procedures were performed under anesthesia with tiletamine chlorohydrate 125 mg/5 mL and zolazepam chlorohydrate 125 mg/5 mL, at a dose of 0.4 mL/kg, in addition to fentanyl citrate + droperidol. Guinea pigs were submitted to a biopsy of left nasal mucosa material using a punch biopsy forceps, obtaining fragments measuring approximately 2 × 2 × 2 mm. All fragments were immersed immediately upon collection in 4% buffered formalin and sent for histologic processing.
Guinea pigs were given 0.5mL of estradiol cypionate, sterile solution, 2mg/mL (E.C.P.TM, Pfizer) intraperitoneally, daily for 30 days. The guinea pigs were given access to feed and water ad libitum. After 30 days of hormonal therapy, the guinea pigs were sacrificed by injection of a lethal dose of potassium chloride under anesthesia, and submitted to exposure and removal of the nasal mucosa of the middle turbinate contralateral to the initial biopsy site.
Immunohistochemical analysis
The tissue samples underwent sequential immersion in buffered formalin for 1h, immersion in absolute alcohol (six times in different recipients, each for 1h), immersion in xylol (four times in different recipients, each for 1h), and immersion in liquid paraffin (twice in different recipients, each for 1 h). Subsequently, specimens were embedded in liquid paraffin. The paraffin blocks for the two time points were sent to the Rodent Histopathology Core of the Pathology Department of Harvard Medical School, Boston, United States, where blocks were cut into 4µm-thick slices and mounted on electrically charged slides, with each slide holding two slices. The slides were then sent to the pathology laboratory of the Brigham and Women's Hospital, Boston, United States, for immunohistochemical processing. In this procedure, the slides were submitted to the deparaffination process by immersion for 10 minutes in Hemo-DeTM (Scientific Safety Solvents - Texas, USA), followed by immersion in six different recipients of absolute alcohol. Endogenous peroxidase blocking was then performed by immersion in a solution constituting 30% volume of 3% hydrogen peroxide and 70% volume of absolute alcohol. The slides were then washed in running water for 3 minutes.
Antigenic recovery was then performed by immersion in buffered pH 6-citrate solution pre-heated to 25ºC for 30 minutes. The sections were delimited using a pap penTM (SciTek Laboratories). Slides were left for 1 minute in a recipient containing 500ml of PBS with 10 mL of 1% BRU. After removal from this solution, the non-specific primary anti-body H222 was applied (marker of both ERα and ERβ) for ERs at a final concentration of 5µg/mL, and submitted to overnight incubation at 4ºC in a wet chamber. Following incubation, slices were rinsed with PBS to remove excess primary antibody and then re-immersed in PBS/BRU 1% solution for 10 minutes. Slices were incubated with the secondary antibody Mouse Envision+TM (DakoCytomation - Glostrup, Denmark) for 30 minutes, rinsed again with PBS, and immersed in a PBS/BRU 1% solution for 10 minutes. Sections were then incubated in a solution of DAB+TM (DakoCytomation), 1 drop per mL of buffer for 10 minutes. Upon removal from incubation, slides were rinsed in running water, counterstained with Mayer's hematoxylin (1.5 minutes), rinsed again, submitted to Scott's water (1 minute), and again washed in running water. Sections were then dehydrated, dried, and mounted with coverslips.
Slides were analyzed pairwise, comparing the images obtained before and after hormone therapy through relative optical density (RODTM) analysis.
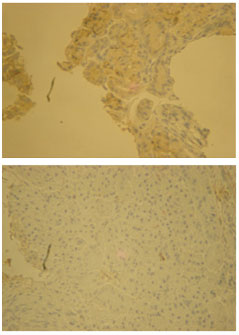
ROD is a software for quantitative imaging assessment that estimates the quantity of solid aggregates in a single bi-dimensional image, after due calibration for a known vertical thickness (immunohistochemistry slide) (Fig.1). The methodology reduces the subjectivity of the observations and enables numerical determination of the tissue stained, facilitating comparison between pairs. For each specimen, four separate pre- and post-therapy slides were assessed, and the average result was used.
Figure 1 Immunohistochemical study for estrogen receptors before (A) and after (B) hormone therapy (40×).
RESULTS
Results were compared using the parametric Student's t-test for paired samples (pre- and post-hormone therapy).
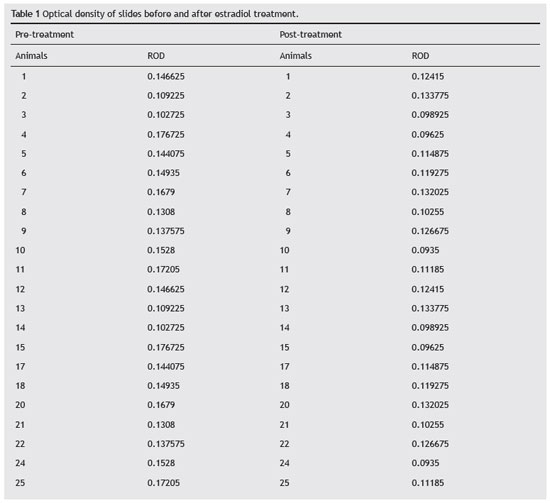
The results of the paired group analysis are shown in Table 1.
Animals 16 and 19 presented no glands in their respective histological slices, whereas the post-therapy slides for animal 23 were lost.
Statistical analysis
The parametric Student's t-test was performed using the ROD's results of samples from 19 animals from pre- and post-therapy slides. The groups were statistically different; the pre-treatment group presented a higher number of receptors than the post-treatment group (p = 0.000005726)
DISCUSSION
Based on clinical observations, a number of studies indicate a correlation between hormonal changes and nasal obstruction. Hormonal rhinitis is cited in various otorhinolaryngology textbooks, yet its underlying physiopathological mechanism remains unclear.
Many studies have already indicated the activity of feminine sexual hormones over the nasal mucosa. It is postulated that, in situations of increased serum hormone (estrogen) levels, there is a stimulus for increased expression of its receptors,34 triggering changes in the density and localization of these receptors.
In the present study, the influence of estradiol administration on the expression of ERs in the nasal mucosa of guinea pigs was observed.
Some issues were observed during the course of the present study, including excessive tissue fragmentation in the pre-estrogen treatment group, which was most likely due to the tissue collection technique employed. The specimens were taken with a small punch biopsy forceps introduced into the nasal cavity. Consequently, the exact site of tissue collection was not precise; besides, the mechanism of action of the instrument (gripping and pulling) may also have led to excessive tissue laceration, evidenced on the slides by a lack of continuity and quality. Additionally, this technique allowed for the collection of only small volumes of material, reducing the quality of histological slices. Conversely, the post-estrogen treatment group had histological slices with higher quality, since the tissue samples were obtained after full surgical exposure of the nasal cavity, enabling the collection of larger amounts of tissue through slicing with a sharp blade. Nevertheless, the fragmentation of the tissue did not compromise the representation of glands on the pre-treatment slides, which showed a good level of immunohistochemical staining.
The suitability of the immunohistochemical staining may also be called into question because the lack of primary antibody activity (H222, non-specific antibody for specific estrogenic receptors for guinea pig species) precluded the assessment of antibody activity, since it was not possible to obtain positive and negative control slides. It is worth remembering that the H222 antibody, specific for the guinea pig species, and which provides non-specific staining of ERs, is no longer commercially available. Geoffrey Greene, who developed the H222 antibody in association with the company Abbott, offered the material used in this study.
The significant reduction of ERs found in animals post-treatment suggests the existence of a negative feedback mechanism triggered by excessive plasma hormone levels. This relationship is poorly discussed in the literature to the degree that the cases of hormonal modification related to nasal reactivity take place during hormonal instability such as pre-menstrual, pregnancy, and menopausal periods.
The expression and stability of the ER is the result of a complex process modulated by estrogens and anti-estrogens. This regulation appears to be the result of two different regulatory mechanisms. Firstly, exposure to estrogen triggers a rapid fall in the mRNA levels and in the proteins responsible for producing ERs. Secondly, exposure to estrogen leads to an increase in the steady state level of mRNA.35
These mechanisms may be absent in women with symptoms of hormonal rhinitis, resulting in the persistence of the receptor level even in the presence of excessive estrogen.
An approach to ascertain the impact of excess estrogen could be to assess the impact of hormone replacement in the ER concentration of the nasal mucosa of post-menopausal women.
Conversely, the negative relationship between estrogen use and the number of nasal ERs indicates a potential utility of topical treatment for the management of rhinitis with a significant glandular component, rhinorrhea, and nasal edema.
CONCLUSION
A significant reduction in the expression of ERs was observed in the nasal mucosa of animals who received estradiol for 30 days.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
1. Scadding GK. Non-allergic rhinitis: diagnosis and management. Curr Opin Allergy Clin Immunol. 2001;1:15-20.
2. Settipane RA, Lieberman P. Update on nonallergic rhinitis. Ann Allergy Asthma Immunol. 2001;86:494-507.
3. Garay R. Mechanisms of vasomotor rhinitis. Allergy. 2004;59:S4-10.
4. Brittebo EB. Localization of oestradiol in the rat nasal mucosa. Acta Pharmacol Toxicol Copenh. 1985;57:285-90.
5. Li N. Effect of over dose sex hormone on rabbit's nasal mucosa. Zhonghua Er Bi Yan Hou Ke Za Zhi. 1991;26:85-7.
6. Zhao XJ, McKerr G, Dong Z, Higgins CA, Carson J, Yang ZQ, et al. Expression of oestrogen and progesterone receptors by mast cellas alone, but nor lymphocytes, macrophages or other immune cells in human upper airways. Thorax. 2001;56:205-11.
7. Dong Z, Zhu J, Sun S. Effect of the purariae-isofiavones on estrogen level in normal and ovariectomized rats. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 1998;12:174-6.
8. Lorenzen I, Schiff M. Vascular connective tissue under the influence of oestrogens. 3. Effect of oestrogens on experimental arteriosclerosis. Morphological and biochemical studies. Acta Endocrinol Copenh. 1968;57:457-64.
9. Schreiber U. Vasomotoric rhinitis as an adverse effect of hormonal contraception. HNO. 1973;21(6):180-1.
10. Armengot M, Basterra J, Marco J. Nasal mucociliary function during the menstrual cycle in healthy women. Rev Laryngol Otol Rhinol. 1990;111:107-9.
11. Haeggstrom A, Ostberg B, Stjerna P, Graf P, Hallen H. Nasal mucosal swelling and reactivity during a menstrual cycle. ORL J Otorhinolaryngol Relat Spec. 2000;62:39-42.
12. Caruso S, Grillo C, Agnello C, Maiolino L, Intelisano G. Serra AA. prospective study evidencing rhinomanometric and olfactometric outcomes in women taking oral contraceptives. Hum Reprod. 2001;16:2288-94.
13. Grillo C, La Mantia I, Triolo C, Scollo A, La Boria A, Intelisano G, et al. Rhinomanometric and olfactometric variations throughout the menstrual cycle. Am Otol Rhinol Laryngol. 2001;110:785-9.
14. Navarrete-Palacios E, Hudson R, Reyes-Guerrero G, Guevara-Guzman R. Correlation between cytological characteristics of the nasal epithelium and the menstrual cycle. Arch Otolaryngol Head Neck Surg. 2003;129:460-3.
15. Philpott CM, Conboy P, Al-Azzawi F, Murty G. Nasal physiological changes during pregnancy. Clin Otolaryngol. 2004;29:343-51.
16. Philpott CM, El-Alami M, Murty GE. The effect of the steroid sex hormones on the nasal airway during the normal menstrual cycle. Clin Otolaryngol. 2004;29:138-42.
17. Mabry RL. Rhinitis of pregnancy. South Med J. 1986;79:965-71.
18. Ellegard EK, The etiology and management of pregnancy rhinitis. Am J Respir Med. 2003;2:469-75.
19. Ellegard EK. Clinical and pathogenetic characteristics of pregnancy rhinitis. Clin Rev Allergy Immunol. 2004;26:149-59.
20. Bende M, Gredmark T. Nasal stuffiness during pregnancy. Laryngoscope. 1999;109:1108-10.
21. Salaroli AF, Fernandez OO, Morandi V, R Júnior LA, Ruston CR, Schmidt JM, et al. Influencia ou não dos anticoncepcionais orais na rinite alergica. J Bras Med. 2001;81:31-4.
22. Toppozada H. The human nasal mucosa in the menopause. J Laryngol Otol. 1988;102:314-8.
23. Toppozada H, Michaels L, Toppozada M, El-Ghazzawi I, Talaat A, Elwany S. The human nasal mucosa in the menstrual cycle. J Laryngol Otol. 1981;95:1237-47.
24. Toppozada H, Michaels L, Toppozada M, El-Ghazzawi I, Talaat A, Elwany S. The human respiratory nasal mucosa in pregnancy. J Laryngol Otol. 1982;96:613-26.
25. Nappi C, Sardo AS, Guerra G, Bifulco G, Testa D, Di Carlo C. Functional and morphologic evaluation of the nasal mucosa before and after hormone therapy in postmenopausal women with nasal symptoms. Fert Steril. 2003;80:669-71.
26. Enmark E, Gustafsson JA. Oestrogen receptors - an overview. J Intern Med. 1999;246:133-8.
27. Ozawa H. Steroid hormones, their receptors and neuroendocrine system. J Nihon Med Sch. 2005;72:316-25.
28. Pavao M, Traish AM. Estrogen receptor antibodies: specifity and utility in detection, localization and analyses of estrogen receptor alpha and beta. Steroids. 2001;66:1-6.
29. Bowser C, Riederer A. Detection of progesterone receptors in connective tissue cells of the lower nasal turbinates in women. Laryngorhinootologie. 2001;80(4):182-6.
30. Balbani APS. Ação do estrógeno e progesterona na mucosa nasal humana: avaliação do transporte mucociliar nasal de sacarina e pesquisa de receptores hormonais através de método imunohistoquímico. [thesis]. Faculdade de Medicas da Universidade de São Paulo: São Paulo; 2001.
31. Millas I. Pesquisa da presença de receptores p/estrógeno tipos Alfa e Beta na mucosa de conchas nasais inferiores através de método imuno-histoquímico. [thesis]. Faculdade de Ciências Médicas da Santa Casa de São Paulo: São Paulo; 2006.
32. Shirasaki H, Watanabe K, Kanaizumi E, Konno N, Sato J, Narita S, et al. Expression and localization of steroid receptors in human nasal mucosa. Acta Otolaryngol. 2004;124:958-63.
33. Millas I, Liquidato BM, Buck HdeS, Barros MD, Paes RA, Dolci JE. Evaluation of estrogenic receptors in the nasal mucosa of women taking oral contraceptives. Contraception. 2011;83:571-7.
34. Levin ER. Minireview: Extranuclear steroid receptors: roles in modulation of cell functions. Mol Endocrinol. 2011;25:377-84.
35. Katzenellenbogen BS, Choi I, Delage-Mourroux R, Ediger TR, Martini PG, Montano M, et al. Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology. J Steroid Biochem Mol Biol. 2000;74:279-85.
Santa Casa de São Paulo, Faculdade de Medicina, São Paulo, SP, Brazil
Corresponding author.
L. Silva
E-mail: leosilva@uol.com.br
Received 29 March 2013.
Accepted 12 October 2013.