Year: 2013 Vol. 79 Ed. 6 - (12º)
Artigo Original
Pages: 720 to 726
Application of the Portuguese version of the Obstructive Sleep Apnea-18 survey to children
Author(s): Fausto Manuel Vigario Santos Fernandes1; Rafaela da Cruz Vieira Veloso Teles2
DOI: 10.5935/1808-8694.20130132
Keywords: child development; health care surveys; sleep apnea, obstructive.
Abstract:
Despite the significant prevalence of obstructive sleep apnea syndrome (OSAS) in children, the diagnosis and treatment of this condition is still challenging due to the difficulties inherent to objectively assessing the disease's severity.
OBJECTIVE: To verify whether the Portuguese version of the Obstructive Sleep Apnea-18 (OSA-18) survey is as valid as its original version in English.
METHOD: Prospective study. The OSA-18 was translated into Portuguese, culturally adapted, and tested in the Portuguese population. The caregivers of 51 children (aged from 2 to 12 years) diagnosed with OSAS answered the OSA-18-pv survey. Statistical analysis was used to assess the psychometric properties of the survey.
RESULTS: Reliability analysis yielded a Cronbach's alpha of 0.821, confirming the survey's consistency. Converging validity was assessed using Pearson's correlation coefficient, which revealed a statistically significant correlation between individual data and total results. The survey can be easily and quickly answered (7.26 min). The outcomes of the OSA-18-pv showed that quality of life was poor in 14 children with OSAS (28%), moderate in 22 (43%), and high in 15 (29%).
CONCLUSION: the OSA-18-pv is a valid tool and can be used to assess the impact on the quality of life of Portuguese children with OSAS.
![]()
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is a respiratory disorder characterized by prolonged partial obstruction and/or intermittent complete obstruction of the upper airways and interruption of normal ventilation during sleep1.
Pediatric OSAS became an important topic not only because of its high prevalence, but also due to its associations with different comorbidities, some of which bearing possible implications to the affected subject's adult life. Moreover, it has been established that the pathophysiology of pediatric OSAS is different from that of OSAS in adult subjects. Although its prevalence is yet unknown, pediatric OSAS has been estimated to affect 1%-2 % of children, with cases peaking between the ages of two and eight years, at a time in which the Waldeyer's ring is enlarged2.
OSAS is the main indication for tonsillectomy and adenoidectomy in children. In the last two decades, a progressive decrease was observed in the number of adenotonsillectomy procedures for recurrent infections and an increase in the number of such procedures for OSAS3. Surgery has been proven effective in controlling neurocognitive sequelae of OSAS, such as poor school performance and attention deficit and hyperactivity disorder, in improving left and right ventricular ejection fraction, and in decreasing the levels of biomarkers of inflammation4. However, pediatric OSAS remains underdiagnosed5 and, therefore, undertreated. Polysomnography (PSG) is currently the gold standard test for the diagnosis of OSAS in children. However, PSG is expensive, time consuming, and not all sleep labs run this test in children. The correct diagnosis and the decision of when to surgically intervene is made difficult due to the limited availability of objective measures to determine the severity of OSAS.
Recently, Franco et al. 6 developed a survey to assess the impact of OSAS in children and their caregivers based on 18 questions. This instrument, called the OSA-18 survey, inquires caregivers in five domains: sleep disturbance, physical symptoms, emotional symptoms, daytime function, and caregiver concerns; each item is given a score from one to seven, in which one means 'never' and seven means 'always'.
The OSA-18 survey proved to be simple and quick to complete, and can be used in clinical or research settings. Its validity for the English-speaking population has been established.
This study aimed to translate, culturally adapt, and validate the Portuguese version of the OSA-18 (OSA-18-pv).
METHOD
Adaptation and translation into Portuguese
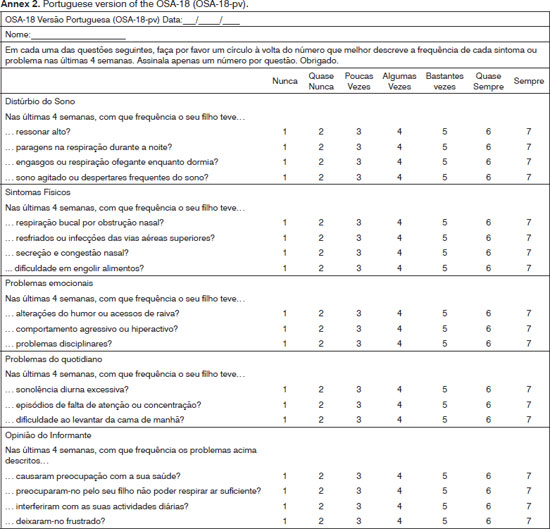
The original version of the OSA-18 survey (Annex 1) was translated by two bilingual physicians into Portuguese, translated back into English, so that then the final version in Portuguese were produced.
Sample and procedures
This study was carried out between March 2011 and March 2012, with patients seen at the Alto Ave Hospital Center in Guimarães, Portugal, for obstructive sleep apnea syndrome. The protocol was approved by the Ethics Committee of the institution. The following enrollment criteria were applied: (1) patients had to be aged between two and 12 years; (2) subjects had to have a history of sleep-disordered breathing suggestive of OSAS for a period exceeding three months. The exclusion criteria were as follows: (1) presence of craniofacial dysmorphic features; (2) patients with psychiatric disorders (except for attention deficit and hyperactivity disorder); or (3) neuromuscular diseases. The questionnaires were completed by the caregivers of children with a physician present in the room as they visited the hospital. A pretest with 10 questionnaires was conducted to assess the difficulties that the survey could present and whether the respondents had properly understood the questions. Some corrections were made considering the Portuguese socioeconomic and cultural context. The final version of the OSA-18 (Annex 2) was therefore applied to the first 51 caregivers of children meeting the enrollment criteria and diagnosed with OSAS.
Statistical analysis
Data was entered into a computer database developed specifically by the IT Department of the hospital. The data sets were processed with statistical analysis software SPSS (Statistical Package for the Social Sciences) version 19 (SPSS, Inc, USA).
Demographic and clinical data sets (age, gender, socioeconomic status according to Graffar7, level of education of caregivers, history of recurrent tonsillitis, Friedman8 classification to evaluate the position of the tongue and the degree of tonsillar hypertrophy, adenoid hypertrophy assessment) were reviewed in the statistical analysis.
The survey's degree of internal consistency (Cronbach's alpha) was validated based on the level of homogeneity seen between the different items of the questionnaire (adequate when > 0.70).
For quantitative variables, a correlation between each individual item and the total score of the OSA-pv-18 was assessed using the Pearson correlation coefficient. Convergent validity is verified when the Pearson correlation coefficient is greater than 0.20.
RESULTS
The sample consisted of 51 Caucasian children, 35 males (69 %) and 16 females (31 %), with a mean age of 5 ± 2 years (2-11 years) with upper and lower quartiles at 4 and 6 years.
According to the Graffar socioeconomic classification, one child belonged to class I (2%), two to Class II (4%), 35 to Class III (69%), 13 to Class IV (25%), and none to Class V.
Two caregivers (4%) graduated from university, three (6%) graduated from high school or two-year degree programs, 11 (22%) did not complete high school or two-year degree programs, and 35 (69%) completed elementary school.
When history of recurrent tonsillitis was considered, 26 children (51%) met the Paradise9 criteria. On physical examination, 31 children (61%) had Friedman grades 3 or 4 tonsillar hypertrophy, while the remaining 20 (39%) had grade 2 tonsillar hypertrophy. The position of the tongue, according to Friedman's classification, was grade I in 18 children (35%) and grade II in the remaining 33 children (65%). Cephalometric evaluation was used to assess the degree of adenoid hypertrophy, which was determined by the ratio between the width of the adenoid tissue and the anteroposterior diameter of the nasopharynx after drawing a line tangential to the spheno-occipital synchondrosis intersecting the soft palate. Fifteen percent of the children had grade 2 adenoid hypertrophy (adenoid-nasopharynx ratios of 26% to 50%), 26% had grade 3 adenoid hypertrophy (ratios of 51% to 75%) and 59% had grade 4 adenoid hypertrophy (ratio > 75%).
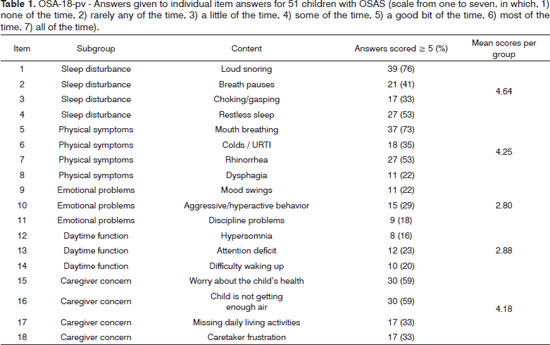
Caregivers answered the surveys while at the hospital in the presence of a physician. The mean time to complete the survey was 7 minutes (4-13 minutes). Five participants (10%) found the questionnaire difficult to understand. On item 'sleep disturbance', the percentages of children who had a score of five or higher (meaning the symptom was present at least 'a good bit of the time') were: 76% for loud snoring; 41% for breath holding spells or pauses in breathing at night; 33% for choking or making gasping sounds while asleep; and 53% for restless sleep or frequent awakenings from sleep. On item 'physical symptoms', mouth breathing because of nasal obstruction was present at least 'a good bit of the time' in 37 children (73%); frequent colds or upper respiratory infections in 18 (35%); nasal discharge or a runny nose in 27 (53%); and difficulty in swallowing foods in 11 (22%). Emotional symptoms (items 9-11) and daytime function (items 12-14) were the two areas with the lowest mean scores. Approximately 59% of the caregivers felt concerned about the health of their children; 59% also felt concern that their children were not breathing enough air; 33% felt that these problems interfered with performance in daily activities; and 33% felt frustrated with the children's condition most of the time (Table 1).
Reliability analysis was carried out on SPSS based on internal consistency, yielding a Cronbach's alpha of
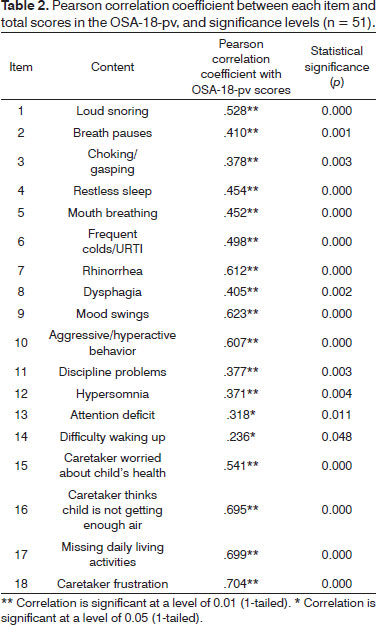
0.821 considering all items in the study. Converging validity was verified through the Pearson correlation coefficient, and each item was seen to have a statistically significant correlation with the OSA-18 total score and a Pearson coefficient correlation above 0.20 (Table 2).
Based on the OSA-18-pv total score, the impact of OSAS on quality of life was mild in 14 cases (28%), moderate in 22 (43%), and severe in 15 (29%) (Table 3).
DISCUSSION
The OSA-18 is a quick, easy-to-use, highly reliable and consistent test used to evaluate the subjective aspects of quality of life in children with OSAS6. This study showed that the OSA-18-pv is an adequate translation of its original version in English, as demonstrated by the high reliability manifested through a Cronbach's alpha of 0.821 and significant consistency illustrated by the correlation between individual test items and total scores, which yielded a Pearson correlation coefficient > 0.20 and p < 0.05. Generally, the caregivers of the children with OSAS found the survey easy to understand; the five caregivers (9.8%) with medium-to-low income levels and low levels of education found the questions difficult to understand.
This survey allows physicians to better assess the impact of OSAS on affected children and their families, in addition to improving the selection from different treatment classes when combined with other clinical parameters and objectives. The survey is a quick test that can be used by physicians of various specialties.
The consistency and reliability of this study can be attributed to the fact that a guiding protocol was used and that the data sets were collected by the same author. Our protocol for Obstructive Sleep Apnea Syndrome10 includes systematic interviews in which caregivers are asked about the nocturnal signs and symptoms related to obstructive disease, including parasomnias, daytime symptoms, signs of adenotonsillar hypertrophy, and cognitive and behavioral problems. The second part of the protocol revolves around the application of the OSA-18-pv to caregivers in order to assess the impact of the disease on their lives. The children also undergo complete physical examination, which includes ENT evaluation, an analysis of their development in terms of height and body weight, and cardiovascular examination.
The most frequently reported symptoms in the OSA-18-pv survey were sleep disturbances (items 1-4), followed by physical symptoms (items 5-9), and caregiver concerns (items 16-18), as similarly reported by other authors6. Emotional problems and daytime function (items 10-15) were the areas with the lowest mean scores, although symptoms were often related to OSAS, according to the literature11, and interfered significantly with the quality of life of patients with OSAS. The OSA-18 also has the advantage of considering the neurobehavioral problems of children, while polysomnography does not take psychological components into account.
CONCLUSION
The OSA-18-pv is a quick, easy-to-use instrument used to determine the quality of life of subjects with OSAS. The survey can be used in future research studies. More scientific research is needed in the area of pediatric OSAS to determine diagnostic criteria, correlate survey data with quality of life, clinical parameters with polysomnography results, to thus determine when it is necessary to carry out polygraphic sleep recordings/polysomnography to allow the timely referral of patients to ENT care and the identification of good candidates for surgery.
REFERENCES
1. Standards and indications for cardiopulmonary sleep studies in children. American Thoracic Society. Am J Respir Crit Care Med. 1996;153(2):866-78.
2. Uema SF, Vidal MV, Fujita R, Moreira G, Pignatari SS. Behavioral evaluation in children with obstructive sleep disorders. Braz J Otorhinolaryngol. 2006;72(1):120-2. PMID: 16917563
3. Rosenfeld RM, Green RP. Tonsillectomy and adenoidectomy: changing trends. Ann Otol Rhinol Laryngol. 1990;99(3 Pt 1):187-91.
4. Church GD. The role of polysomnography in diagnosing and treating obstructive sleep apnea in pediatric patients. Curr Probl Pediatr Adolesc Health Care. 2012;42(1):2-25. DOI: http://dx.doi.org/10.1016/j.cppeds.2011.10.001
5. Meltzer LJ, Johnson C, Crosette J, Ramos M, Mindell JA. Prevalence of diagnosed sleep disorders in pediatric primary care practices Pediatrics. 2010;125(6):e1410-8.
6. Franco RA Jr, Rosenfeld RM, Rao M. First place--resident clinical science award 1999. Quality of life for children with obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000;123(1 Pt 1):9-16. PMID: 10889473
7. Graffar M. Une methode de classification sociale d'echantillons de population. Courrier. 1956;6(8):455-9.
8. Friedman M. Sleep Apnea and Snoring: surgical and non-surgical therapy. Philadelphia: Saunders-Elsevier; 2008.
9. Paradise JL, Bluestone CD, Bachman RZ, Colborn DK, Bernard BS, Taylor FH, et al. Efficacy of tonsillectomy for recurrent throat infection in severely affected children. Results of parallel randomized and nonrandomized clinical trials. N Engl J Med. 1984;310(11):674-83. DOI: http://dx.doi.org/10.1056/NEJM198403153101102
10. Veloso-Teles R, Estevão R, Caselhos S, Moreira-Silva F, Fernandes F. Elaboração de um Protocolo Orientador da Consulta de SAOS da Criança. Rev Portuguesa Otorrinolaringol. 2011 (artigo submetido).
11. O'Brien LM, Mervis CB, Holbrook CR, Bruner JL, Klaus CJ, Rutherford J, et al. Neurobehavioral implications of habitual snoring in children. Pediatrics. 2004;114(1):44-9. DOI: http://dx.doi.org/10.1542/peds.114.1.44
1. Head of the ENT Service (Head of the Hospital ENT Service)
2. Bachelor's Degree in Medicine (ENT Intern, Alto Ave Hospital Center, Guimarães)
Otorhinolaryngology Service of the Alto Ave. Hospital Center.
Send correspondence to:
Fausto Fernandes
Rua dos Cutileiros, Creixomil, 4835-044
Guimarães. Portugal
Telefone: 00351 253 540 330
E-mail: faustoorl@gmail.com
Paper submitted to the BJORL-SGP (Publishing Management System - Brazilian Journal of Otorhinolaryngology) on July 26, 2013.
Accepted on August 27, 2013. cod. 11030.