Year: 2013 Vol. 79 Ed. 5 - (19º)
Artigo de Revisão
Pages: 636 to 642
Surgical treatment and adjuvant therapies of recurrent respiratory papillomatosis
Author(s): Melissa Ameloti Gomes Avelino1; Tallyta Campos Domingues Teixeira Zaiden2; Raquel Oliveira Gomes2
DOI: 10.5935/1808-8694.20130114
Keywords: larynx; papilloma; papillomavirus infections; papillomavirus vaccines; therapeutics.
Abstract:
Recurrent respiratory papillomatosis or recurrent laryngeal papillomatosis is a disease of the larynx caused by human papilloma virus, characterized by verrucous epithelial lesions and usually recurring. In the literature there are several types of treatment, such as surgery to cold, laser and/or use of microdebrider, as of adjuvant therapies; all possible to decrease the permanent sequelae of the disease.
OBJECTIVE: To review the literature regarding this disease with emphasis on surgical techniques and adjuvant therapies used today.
METHOD: We used the literature review, through surveys based electronic data in the public domain, to search for articles between 1992-2012, using keywords: papilloma, human pappiloma virus infection, larynx, therapeutic, papilloma virus vaccine.
RESULTS: We surveyed 357 articles, of which 49 were used as the basis for this review. Scientific studies indicate a reduction of relapse in most adjuvant therapeutic presented. However, the survey showed different methodologies and samples, which did not allow to compare the types of treatment and adjuvant therapies.
CONCLUSION: The choice of surgical technique varies among studies, but there is a trend to use the microdebrider. The newer adjuvant therapies, such as cidofovir, quadrivalent vaccine against human papilloma virus and bevacizumab, require further studies.
![]()
INTRODUCTION
Recurrent respiratory papillomatosis (RRP) or laryngeal papillomatosis is a laryngeal disease caused by the human papilloma virus characterized by the proliferation of recurring epithelial wart-like lesions called papillomas. Papillomas commonly relapse and occur in significant numbers1,2. RRP is the most frequently seen benign laryngeal tumor. Confluent papillomas introduce significant morbidity and may lead to dysphonia (voice alteration) and dyspnea (respiratory pattern alteration)2,3.
RRP results from infection by the human papilloma virus (HPV). The most often detected virus subtypes are HPV-6 and HPV-11, seen in approximately 90% of the cases1,2. HPV-16 and HPV-18 are found more rarely in children with RRP3, and have been more strongly correlated with malignant disease.
The mode of transmission of HPV has not been clearly established. Although transmission by sexual intercourse has been described, possible nonsexual routes include vertical and horizontal transmission, and autoinoculation. A recent meta-analysis showed that vertical transmission accounts for approximately 20% of the cases of HPV. However, horizontal transmission through saliva or other means has also been described2,4. Upper airway exposure to HPV-6 and HPV-11 occurs somewhat frequently during one's life. It is likely that exposure to HPV is as common as exposure to other viruses, as studies have detected HPV DNA in the upper airways of as many as 25% of unaffected children and adults5.
According to Derkay6, some 1,500 to 2,500 new cases of RRP are diagnosed in the United States every year. Tasca et al.7 have reported an incidence of 6.9 cases for every 1,000 babies born from mothers with history of genital warts, against no cases per 1,000 births of children from mothers without history of genital warts. The authors have also reported that risk of RRP in children born from mothers with papillomas during pregnancy is 231.4 times higher than in mothers without history of papilloma. Incidence rates have been estimated at 4.3 cases for every 100,000 children and 1.8 case for every 100,000 adult subjects6.
The disease is usually categorized according to the severity of the signs and symptoms, and is divided into aggressive and non-aggressive RRP. Aggressive RRP is characterized by the need for ten or more surgical procedures, with three or more procedures being performed within the period of one year, or by when the disease extends distally toward the subglottic airway. Contrastingly, non-aggressive RRP is characterized by the need for fewer than ten procedures, with fewer than three procedures being performed within the period of one year, or no distal involvement reaching the subglottic area8.
Diagnosis is performed with the aid of fibroscopy (flexible scope) and laryngoscopy (rigid scope) and confirmation is attained through pathology testing. Macroscopically, laryngeal papillomas are pedunculated uneven nodular tumors of varying sizes. In terms of histology, papillomas are extremely vascularized, often keratinized tumors made of connective tissue covered by stratified squamous epithelium that project outwardly in finger-like fronds9.
Treatment is designed to maintain airway patency, improve patient voice quality, and prevent against complications. Surgery is the therapy of choice for RRP. Cold microsurgery, laser microsurgery, or surgery with a microdebrider are used to spare healthy tissue, prevent the formation of scar tissue, and remove all papillomas1,10. Nonetheless, surgery cannot prevent recurrence, thus opening a precedent for the use of adjuvant therapies.
Adjuvant therapies have been given significant attention in the realm of RRP care. Many treatments have been described in the literature, but none appear to provide patients with a cure. Palliative care has been offered in the form of interferon-alpha, indole-3-carbinol, photodynamic therapy, cis-retinoic acid, cidofovir, aciclovir, ribavirin, bevacizumab, and tetravalent HPV vaccine6-19.
There still is no consensus on the best course of adjuvant therapy. This study aimed to review the literature on RRP and focus on the most frequently used surgical approaches and adjuvant therapies.
METHOD
The literature review was carried out based on the following question: 'what are the surgical procedures and adjuvant therapies currently used in the treatment of recurrent respiratory papillomatosis?'. The search was carried out on databases Cochrane, LILACS (Literature in the Health Sciences in Latin America and the Caribbean), MedLine (USA National Library of Medicine), PubMed, and SciELO for papers written between January of 1992 and November of 2012. The following keywords were used in the search for papers: papilloma, papillomavirus infection, larynx, therapy, papillomavirus vaccines. Only papers written in Portuguese, Spanish, and English were included.
The papers found in the initial search were independently assessed by two authors. In order not to exclude important studies from the review, after a consensus meeting the two reviewers selected all potentially relevant papers accompanied or not by their abstracts. After the selection of relevant titles, papers in full were collected and assessed based on a protocol considering the following topics: type of study, sample, adopted approach, and reported results. All cross-sectional, longitudinal, prospective, and retrospective clinical trials, review papers, meta-analyses, case reports, and chapters published in Brazilian books meeting the criteria were included. Papers with significantly similar contents were excluded, and priority was given to the first authors to publish on the topic on Brazil or abroad, and/or to papers with larger and more recent samples. Included studies were chosen based on how well they answered the guiding question of this literature review. The book chapter selected for this review was chosen from a recent publication of 2011.
RESULTS
Forty-nine of the 357 papers listed in the search result were included in this literature review. Most authors reported less recurrence with surgery and adjuvant therapy combined. However, the differences in materials and methods did not allow comparisons between types of surgical treatment and adjuvant therapies.
Authors agree that surgical removal of papillomas is the treatment of choice for patients with papillomatosis, despite differences between approaches. Larynx microsurgery can be performed with cold instruments, laser, and, as reported more recently, microdebriders. This review revealed that laryngeal sequelae were reported in 6% to 61% of the cases, depending on the chosen procedure. More recent studies have preferred microdebriders to other surgical instruments.
Drugs such as interferon, often described in the past, have not been used as adjuvant therapy in recent papers, indicating a trend toward the discontinuation of this type of therapy.
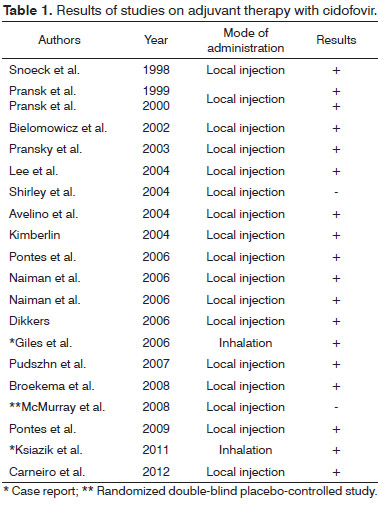
Although most recent papers still prefer cidofovir in the adjuvant therapy of RRP, some studies have failed to report significant improvements consequent to the use of this medication. In early studies, cidofovir was indicated only in local or intralesional treatment. Some authors have also described the use of inhaled cidofovir (Table 1).
The papers written by Zeitels et al. as of 2009 have described very positive results for bevacizumab in association with KTP laser as an adjuvant therapy14,15. However, the low number of studies on Avastin, the samples, and methods used in these studies do not allow conclusions to be drawn on the effectiveness of this RRP adjuvant therapy.
Although the HPV vaccine has been indicated to prevent against infection by the four subtypes of HPV (6, 11, 16, and 18), it has also been used as adjuvant therapy in patients with RRP.
In 2008, Förster et al.20 suggested the vaccine was beneficial for RRP patients and called for the organization of a multicenter trial. In 2009, Pawlita & Gissmann21 recommended papillomatosis adjuvant therapy with tetravalent HPV vaccine. In 2011, Mudry et al.17 reported the case of a five-year-old submitted to multiple procedures for frequently recurring papillomas followed up for 17 months who entered remission after taking the HPV vaccine. However, this therapy has been described in the literature merely in the form of isolated case reports.
DISCUSSION
Disease progress varies in RRP. While some patients with laryngeal papillomatosis see their condition regress spontaneously, others require multiple procedures for decades, with recurrence taking place within less than two weeks of surgery. Children usually experience more aggressive relapses and have less encouraging prognosis1,2. The treatment of RRP can be a significant challenge in ENT practice. Although surgery is the treatment of choice, it does not prevent lesions from recurring.
Surgery aims to preserve uninvolved tissue, completely remove papillomas, and avoid scarring. It can be performed with cold instruments, CO2 laser, or microdebriders1,2,10. New approaches to surgery have been developed since cold laryngeal microsurgery. In the early 1970s, the advent of laser technology gave birth to a new surgical approach believed to offer the following advantages: better control over bleeding, higher accuracy, and longer disease-free periods. Thus, this approach was chosen as the first-line treatment for RRP20-24.
However, the indications and use of CO2 laser have been challenged in recent years by a number of authors. Studies have revealed the procedure presents higher risk of respiratory tract burns, and is more prone to producing stenosis and severe laryngeal scarring, distal injuries with tracheoesophageal fistulae, in addition to increasing the cost of treatment when compared to surgery with a microdebrider2,25,26. Other authors, such as Morgan e Crockett, reported complication rates of 61% and 36% with laser surgery, respectively1,2. A retrospective study on the complications of CO2 laser surgery found that 10 of the 17 enrolled subjects had laryngeal scarring27. In 2007, Preuss et al.12 reported complication rates of only 6% in laser surgery against 20% when using the conventional approach.
In this context, microdebrider surgery has conquered adepts and is gradually replacing laser surgery as the surgical procedure of choice. Some authors have pointed out advantages such as shorter time of surgery, lower cost, lower risk of complications, and better patient voice quality when compared to CO2 laser2,12,23. The risk of sequelae is believed to vary depending on the type of procedure the patients are offered. Therefore, while some authors credit laser as the culprit of cases of laryngeal scarring, others prefer to defend its use. Nonetheless, regardless of the approach/instruments used, the virus remains latent even after careful resection, thus leading to recurrence1. Managing recurrence often requires extensive manipulation of the larynx, and possibly leads to permanent damage in the form of stenosis, anterior and posterior membrane neoformation, vocal fold injury, and granulation tissue formation, among others2. A retrospective study with 50 children with RRP revealed that laryngeal sequelae occur frequently, particularly anterior commissure synechia and glottic stenosis1 .
Adjuvant therapies gained ground in medical practice as an attempt to minimize recurrence, its complications, the negative impacts it has upon patients' lives, and reduce or eliminate the need for future surgery. The most widely accepted criteria to start adjuvant therapy are: need for more than four surgical procedures per year, lesions extending toward the subglottic region, and short disease-free intervals leading to airway involvement28. Between 12.6% and 47.6% of the children with RRP are given adjuvant therapy7,10,18,29, as recommended in cases of aggressive disease with frequently recurring papillomas or distal involvement of the airways10,30.
Interferon-alpha was the first medication offered as adjuvant therapy to RRP patients. Although it is still used today, interferon-alpha is no longer as popular as it once was. In its early days, the drug seemed to produce good outcomes, particularly within the first few months of use, but patients experienced multiple side effects. Other therapies and drugs such as indole-3-carbinol, photodynamic therapy, and cis-retinoic acid were studied, but none grew to become the therapy of choice for subjects with RRP3,10.
In recent years, antiviral drug cidofovir [(S)-1-[(3-hydroxy-2-phosphonyl methoxy) propyl] cytosine (HPMPC )] was introduced as a potent replication inhibitor for HPV, adenovirus, cytomegalovirus, and herpesvirus2,10,31-41.
Cidofovir has been used as an adjuvant drug since 1998. In 1998, Snoeck et al. studied the use of cidofovir injections to control RRP in adult subjects, while Pransky et al. analyzed its impact on pediatric populations in 1999 and 200031-33. The drug is a cytosine analog and a potent inhibitor of viral replication. Nephrotoxicity is a significant, frequently seen side effect described in patients on cidofovir. Other adverse effects include neutropenia, weakness, nausea, and diarrhea10. However, a study on the local administration of cidofovir to treat children with RRP did not report alterations in their lab workup or adverse effects to the medication, apparently reaffirming the drug's safety when administered locally3,7,32-39. The literature offers a vast array of papers on cidofovir. However, favorable outcomes (remission or less severe disease in most patients)33-39 have been reported along with no significant improvements in disease recurrence40,41.
Cidofovir was described as the most used adjuvant therapy on a survey carried out with members of the American Society of Pediatric Otolaryngology, with interferon ranking second. Sixty-one percent of the patients followed up in the services of the physicians included in the survey improved or were disease-free after treatment, against only four percent of cases in which the disease became more severe. Nonetheless, this study may contain bias and sampling errors, in addition to having used a subjective assessment tool to analyze patient responses, which may have affected the published results18.
A study carried out by members of the British Association of Paediatric Otorhinolaryngology in 2006 showed that cidofovir was the most frequently prescribed drug in the treatment of individuals with RRP. However, the study failed to determine the role of cidofovir in the treatment or to quantify decreases in recurrence7. Some authors began to wonder whether intralesional cidofovir would increase the potential for the development of malignant disease. The Food and Drug Administration (FDA) has considered cidofovir to be possibly carcinogenic, once it increased the incidence of breast adenocarcinoma in rats42. Intralesional cidofovir has also been connected to cases of dysplasia in human beings43-45. Although Broekema reported malignant degeneration in a group of patients given 2.7% cidofovir, the author pointed out that spontaneous malignant degeneration occurs in 2-3% of RRP patients, thus ruling out the alleged association between cidofovir and increased incidence of malignant disease. All the more, HPV increases the chance of development of malignant disease alone, independently from cidofovir43.
In 2008, Lindsay et al.13 analyzed the histology of papillomas before and after the administration of cidofovir and did not observe the occurrence of dysplasia in the patients. The author also reported that cidofovir could potentially cause vocal fold scarring, and suggested the drug should be used with caution on the vocal folds13.
Only case reports have described the use of inhaled cidofovir in the literature. In 2006, a report was published in which inhaled cidofovir was used to reach lung lesions43,46. It described the case of an eleven-month-old girl with respiratory failure and stridor (due to RRP) who underwent a tracheostomy. In order to reach papillomas in the lungs, cidofovir was administered with the aid of a nebulizer connected to the tracheostomy tube. The only reported complication was hemoptysis, which resolved as the drug dosage was reduced. The patient responded well, and her lower airway lesions shrank to a size invisible to the naked eye and the complications disappeared46.
In 2011, another report described the case of a previously healthy four-month-old with weak cry, hoarseness, and increased respiratory effort. Examination with a flexible fiberscope revealed the patient had papillomas in the glottis, which were removed with a microdebrider. Despite treatment with a microdebrider every two weeks, intralesional cidofovir, and systemic interferon-alpha, the patient's health deteriorated substantially from persistent complications. The patient was then offered inhaled cidofovir after the failure of conventional therapies. Significant improvement was seen after six weeks of treatment. Nonetheless, further studies are needed to look into the long-term effectiveness of the treatment and the safety profile of inhaled cidofovir47.
According to this literature review, although cidofovir is the most frequently used adjuvant therapy, it is not universally accepted. The need to find alternative RRP adjuvant therapies has recently led to the development of new treatment modes with bevacizumab and tetravalent HPV vaccine.
Considering the use of bevacizumab (Avastin), this review showed that vascularization is believed to be a determining factor in how quickly papillomas reappear. Thus, bevacizumab would work as an angiogenesis inhibitor, to hamper or stop the growth of papillomas and associated complications15,16. Complementary modes of action and reported safety have led authors to advocate the combination of local injections of bevacizumab and KTP laser surgery. The clinical successes of bevacizumab and KTP laser reported within the last two years and the lack of complications after over 200 laryngeal injections of bevacizumab have pushed this therapy to the top of the list of RRP treatment options15,16. However, larger and more significant studies are required to prove the benefits of this therapy. Relevant questions have been posed about the combined use of bevacizumab and KTP laser. Is activation by laser needed for bevacizumab to yield proper benefit? Could the drug be used without KTP laser?
Tetravalent HPV vaccine protects against viral subtypes 6, 11, 16, and 18 and works on an acquired immunity mechanism characterized by specificity and memory; it is mediated by B-cells, T-cells, antibodies, and cytokines48,49. When an antigen gets in touch with these cells, a specific response is produced. Then, the antigen produces a strong qualitative and quantitative response, with high levels of antibodies, thus configuring the immune memory and long-term protection. Immunogenicity, the ability to provoke a specific immune response, is usually measured as a function of antibody counts48. Tetravalent HPV vaccine is a killed vaccine, which does not contain antigens able to replicate in the individuals given the shot. It is produced using molecular biology techniques. Tetravalent HPV vaccine contains particles produced in vitro from viral proteins which are similar to the virus. They combine spontaneously to form structures similar to the whole virus48,49. It is important to note that the vaccine does not have infectious or oncogenic potential, and that it is very safe. Clinical trials currently in progress have not reported significant adverse events; side effects were limited to pain, redness, and edema on the site of injection, low fever, headache, and syncope48.
Although vaccination is currently considered only for patients not infected by HPV, it has also been studied for patients with RRP. In 2008, Förster reported the case of a two-year-old with aggressive laryngeal papillomatosis. After three shots of tetravalent HPV injection, the patient's condition was stabilized without surgery 10 months into follow-up2,20.
In 2009, Pawlita & Gissmann described the use of tetravalent HPV vaccine as an adjuvant therapy for papillomatosis, reinforcing the role this medication could have in infected patients and the very low risks associated with its use2,21.
In 2011, Mudry et al. reported the case of a five-year-old with frequent recurring papillomas submitted to multiple procedures. The patient entered remission after the administration of tetravalent HPV vaccine. The 17 months for which this patient has been disease-free ranks as one of the longest periods without disease reported in the literature2,17.
Many case report authors have described changes to the natural course of the disease, stabilization, or significant reductions in papilloma recurrence after vaccination. However, as these are only isolated case reports, comprehensive multicenter trials are required to assess the true benefits of vaccination in the treatment of RRP16,17,20,21.
This literature review indicated that the use of surgery and adjuvant therapies largely depends on the context of each service, the experience of the physicians involved, the possible adverse effects and, mainly, the acceptance of patients, and not only on how effective the treatment can be.
CONCLUSION
This literature review showed there is no consensus around one surgical approach for RRP, despite a recent trend toward the use of microdebriders. In regards to adjuvant therapies, although cidofovir is the most frequently used drug, large multicenter trials are required to better describe the levels of efficacy offered by this drug.
REFERENCES
1. Hermann JS, Pontes P, Weckx LL, Fujita R, Avelino M, Pignatari SS. Laryngeal sequelae of recurrent respiratory papillomatosis surgery in children. Rev Assoc Med Bras. 2012;58(2):204-8.
2. Avelino MAG, Ferri RG. Conduta no papiloma laríngeo. In: Devitis RA, Tsuji DH, editores. Manual Prático de Laringologia. 1a ed. Rio de Janeiro: Di Livros Editora; 2011. p.177-84.
3. Avelino MAG, Gutzman RL, Fujita RR, Pignatari S, Weckx LLM, Pontes P. Estudo do efeito do Cidofovir na papilomatose laríngea recorrente em crianças: relato preliminar. Rev Bras Otorrinolaringol. 2004;70(6):734-8. DOI: http://dx.doi.org/10.1590/S0034-72992004000600003
4. Syrjänen S. Current concepts on human papillomavirus infections in children. APMIS. 2010;118(6-7):494-509. PMID: 20553530
5. Lee JH, Smith RJ. Recurrent respiratory papillomatosis: pathogenesis to treatment. Curr Opin Otolaryngol Head Neck Surg. 2005;13(6):354-9. DOI: http://dx.doi.org/10.1097/01.moo.0000186205.91332.46
6. Derkay CS. Recurrent respiratory papillomatosis. Laryngoscope. 2001;111(1):57-69. PMID: 11192901 DOI: http://dx.doi.org/10.1097/00005537-200101000-00011
7. Tasca RA, McCormick M, Clarke RW. British Association of Paediatric Otorhinolaryngology members experience with recurrent respiratory papillomatosis. Int J Pediatr Otorhinolaryngol. 2006;70(7):1183-7. PMID: 16414125 DOI: http://dx.doi.org/10.1016/j.ijporl.2005.12.003
8. Doyle DJ, Gianoli GJ, Espinola T, Miller RH. Recurrent respiratory papillomatosis: juvenile versus adult forms. Laryngoscope. 1994;104(5 Pt 1):523-7.
9. Bailey BJ, Calhoun KH, Derkay CS, Friedman N, Gluckman J, Healy GB, etal. Recurrent Respiratory Papillomatosis. In: Bailey BJ, Calhoun KH, Derkay CS, Friedman N, Gluckman J, Healy GB, et al., eds. Head and Neck Surgery - Otolaryngology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2001. p.659-65.
10. Pontes P, Weckx LLM, Pignatari SSN, Fujita RR, Avelino MAG, Sato J. Aplicação local de cidofovir como tratamento adjuvante na papilomatose laríngea recorrente em crianças. Rev Assoc Med Bras. 2009;55(5):581-6. DOI: http://dx.doi.org/10.1590/S0104-42302009000500023
11. Goon P, Sonnex C, Jani P, Stanley M, Sudhoff H. Recurrent respiratory papillomatosis: an overview of current thinking and treatment. Eur Arch Otorhinolaryngol. 2008;265(2):147-51. PMID: 18046565 DOI: http://dx.doi.org/10.1007/s00405-007-0546-z
12. Preuss SF, Klussmann JP, Jungehulsing M, Eckel HE, Guntinas-Lichius O, Damm M. Long-term results of surgical treatment for recurrent respiratory papillomatosis. Acta Otolaryngol. 2007;127(11):1196-201. PMID: 17851940 DOI: http://dx.doi.org/10.1080/00016480701200350
13. Lindsay F, Bloom D, Pransky S, Stabley R, Shick P. Histologic review of cidofovir-treated recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol. 2008;117(2):113-7. PMID: 18357834
14. Zeitels SM, Lopez-Guerra G, Burns JA, Lutch M, Friedman AM, Hillman RE. Microlaryngoscopic and office-based injection of bevacizumab (Avastin) to enhance 532-nm pulsed KTP laser treatment of glottal papillomatosis. Ann Otol Rhinol Laryngol Suppl. 2009;201:1-13.
15. Zeitels SM, Barbu AM, Landau-Zemer T, Lopez-Guerra G, Burns JA, Friedman AD, et al. Local injection of bevacizumab (Avastin) and angiolytic KTP laser treatment of recurrent respiratory papillomatosis of the vocal folds: a prospective study. Ann Otol Rhinol Laryngol. 2011;120(10):627-34. PMID: 22097147
16. Ramet J, van Esso D, Meszner Z.; European Academy of Paediatrics Scientific Working Group on Vaccination. Position paper--HPV and the primary prevention of cancer; improving vaccine uptake by paediatricians. Eur J Pediatr. 2011;170(3):309-21. DOI: http://dx.doi.org/10.1007/s00431-010-1265-9
17. Mudry P, Vavrina M, Mazanek P, Machalova M, Litzman J, Sterba J. Recurrent laryngeal papillomatosis: successful treatment with human papillomavirus vaccination. Arch Dis Child. 2011;96(5):476-7. PMID: 21220258 DOI: http://dx.doi.org/10.1136/adc.2010.198184
18. Schraff S, Derkay CS, Burke B, Lawson L. American Society of Pediatric Otolaryngology members' experience with recurrent respiratory papillomatosis and the use of adjuvant therapy. Arch Otolaryngol Head Neck Surg. 2004;130(9):1039-42. DOI: http://dx.doi.org/10.1001/archotol.130.9.1039
19. Silverberg MJ, Thorsen P, Lindeberg H, Grant LA, Shah KV. Condyloma in pregnancy is strongly predictive of juvenile-onset recurrent respiratory papillomatosis. Obstet Gynecol. 2003;101(4):645-52. PMID: 12681865 DOI: http://dx.doi.org/10.1016/S0029-7844(02)03081-8
20. Förster G, Boltze C, Seidel J, Pawlita M, Müller A. Juvenile laryngeal papillomatosis--immunisation with the polyvalent vaccine gardasil. Laryngorhinootologie. 2008;87(11):796-9. PMID: 18759217
21. Pawlita M, Gissmann L. Recurrent respiratory papillomatosis: indication for HPV vaccination? Dtsch Med Wochenschr. 2009;134 Suppl 2:S100-2.
22. Silva AMTC, Cruz AD, Silva CC, Borges FR, Curado MP. Genotipagem de Papiloma Vírus Humano em paciente com papilomatose laríngea recorrente. Rev Bras Cancerol. 2003;49(3):167-74.
23. Lee JH, Smith RJ. Recurrent respiratory papillomatosis: pathogenesis to treatment. Curr Opin Otolaryngol Head Neck Surg. 2005;13(6):354-9. DOI: http://dx.doi.org/10.1097/01.moo.0000186205.91332.46
24. Dedo HH, Yu KC. CO(2) laser treatment in 244 patients with respiratory papillomas. Laryngoscope. 2001;111(9):1639-44. PMID: 11568620 DOI: http://dx.doi.org/10.1097/00005537-200109000-00028
25. El-Bitar MA, Zalzal GH. Powered instrumentation in the treatment of recurrent respiratory papillomatosis: an alternative to the carbon dioxide laser. Arch Otolaryngol Head Neck Surg. 2002;128(4):425-8. PMID: 11926919 DOI: http://dx.doi.org/10.1001/archotol.128.4.425
26. Patel N, Rowe M, Tunkel D. Treatment of recurrent respiratory papillomatosis in children with the microdebrider. Ann Otol Rhinol Laryngol. 2003;112(1):7-10. PMID: 12537050
27. Saleh EM. Complications of treatment of recurrent laryngeal papillomatosis with the carbon dioxide laser in children. J Laryngol Otol. 1992;106(8):715-8. DOI: http://dx.doi.org/10.1017/S0022215100120663
28. Carneiro CG, Neto AF, Filho JAX, Imamura R, DH Tsuji, Sennes UL. Laryngeal Papillomatosis: Preliminary Results with Intralesional Injections of Cidofovir. Int Arch Otorhinolaryngol [Acesso 15 de julho de 2012]. Disponível em: http://www.internationalarchivesent.org/conteudo/acervo_port.asp?id=265
29. Reeves WC, Ruparelia SS, Swanson KI, Derkay CS, Marcus A, Unger ER. National registry for juvenile-onset recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg. 2003;129(9):976-82. PMID: 12975271 DOI: http://dx.doi.org/10.1001/archotol.129.9.976
30. Derkay CS, Wiatrak B. Recurrent respiratory papillomatosis: a review. Laryngoscope. 2008;118(7):1236-47. PMID: 18496162 DOI: http://dx.doi.org/10.1097/MLG.0b013e31816a7135
31. Snoeck R, Wellens W, Desloovere C, Van Ranst M, Naesens L, De Clercq E, et al. Treatment of severe laryngeal papillomatosis with intralesional injections of cidofovir [(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine]. J Med Virol. 1998;54(3):219-25. DOI: http://dx.doi.org/10.1002/(SICI)1096-9071(199803)54:3<219::AID-JMV13>3.0.CO;2-C
32. Pransky SM, Magit AE, Kearns DB, Kang DR, Duncan NO. Intralesional cidofovir for recurrent respiratory papillomatosis in children. Arch Otolaryngol Head Neck Surg. 1999;125(10):1143-8. PMID: 10522508 DOI: http://dx.doi.org/10.1001/archotol.125.10.1143
33. Pransky SM, Brewster DF, Magit AE, Kearns DB. Clinical update on 10 children treated with intralesional cidofovir injections for severe recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg. 2000;126(10):1239-43. PMID: 11031411 DOI: http://dx.doi.org/10.1001/archotol.126.10.1239
34. Bielamowicz S, Villagomez V, Stager SV, Wilson WR. Intralesional cidofovir therapy for laryngeal papilloma in an adult cohort. Laryngoscope. 2002;112(4):696-9. DOI: http://dx.doi.org/10.1097/00005537-200204000-00019
35. Pransky SM, Albright JT, Magit AE. Long-term follow-up of pediatric recurrent respiratory papillomatosis managed with intralesional cidofovir. Laryngoscope. 2003;113(9):1583-7. PMID: 12972938 DOI: http://dx.doi.org/10.1097/00005537-200309000-00032
36. Lee AS, Rosen CA. Efficacy of cidofovir injection for the treatment of recurrent respiratory papillomatosis. J Voice. 2004;18(4):551-6. DOI: http://dx.doi.org/10.1016/j.jvoice.2003.07.007
37. Pontes P, Avelino M, Pignatari S, Weckx LL. Effect of local application of cidofovir on the control of recurrences in recurrent laryngeal papillomatosis. Otolaryngol Head Neck Surg. 2006;135(1):22-7. PMID: 16815177 DOI: http://dx.doi.org/10.1016/j.otohns.2005.10.060
38. Naiman AN, Ayari S, Nicollas R, Landry G, Colombeau B, Froehlich P. Intermediate-term and long-term results after treatment by cidofovir and excision in juvenile laryngeal papillomatosis. Ann Otol Rhinol Laryngol. 2006;115(9):667-72. PMID: 17044537
39. Naiman AN, Abedipour D, Ayari S, Fresnel E, Coulombeau B, Bour JB, et al. Natural history of adult-onset laryngeal papillomatosis following multiple cidofovir injections. Ann Otol Rhinol Laryngol. 2006;115(3):175-81. PMID: 16572605
40. Peyton Shirley W, Wiatrak B. Is cidofovir a useful adjunctive therapy for recurrent respiratory papillomatosis in children? Int J Pediatr Otorhinolaryngol. 2004;68(4):413-8. PMID: 15013606 DOI: http://dx.doi.org/10.1016/j.ijporl.2003.11.007
41. McMurray JS, Connor N, Ford CN. Cidofovir efficacy in recurrent respiratory papillomatosis: a randomized, double-blind, placebo-controlled study. Ann Otol Rhinol Laryngol. 2008;117(7):477-83. PMID: 18700421
42. Kimberlin DW. Current status of antiviral therapy for juvenile-onset recurrent respiratory papillomatosis. Antiviral Res. 2004;63(3):141-51. PMID: 15451182
43. Broekema FI, Dikkers FG. Side-effects of cidofovir in the treatment of recurrent respiratory papillomatosis. Eur Arch Otorhinolaryngol. 2008;265(8):871-9. PMID: 18458927 DOI: http://dx.doi.org/10.1007/s00405-008-0658-0
44. Dikkers FG. Treatment of recurrent respiratory papillomatosis with microsurgery in combination with intralesional cidofovir--a prospective study. Eur Arch Otorhinolaryngol. 2006;263(5):440-3. PMID: 16328406 DOI: http://dx.doi.org/10.1007/s00405-005-1013-3
45. Pudszuhn A, Welzel C, Bloching M, Neumann K. Intralesional Cidofovir application in recurrent laryngeal papillomatosis. Eur Arch Otorhinolaryngol. 2007;264(1):63-70. PMID: 17058091 DOI: http://dx.doi.org/10.1007/s00405-006-0151-6
46. Giles BL, Seifert B. CR12/339--Nebulized cidofovir for recurrent respiratory papillomatosis: a case report. Paediatr Respir Rev. 2006;7 Suppl 1:S330.
47. Ksiazek J, Prager JD, Sun GH, Wood RE, Arjmand EM. Inhaled cidofovir as an adjuvant therapy for recurrent respiratory papillomatosis. Otolaryngol Head Neck Surg. 2011;144(4):639-41. PMID: 21493248 DOI: http://dx.doi.org/10.1177/0194599810395353
48. Vespa Junior N. Vacina Quadrivalente contra HPV 6, 11, 16, 18: a Mais Nova Ferramenta de Prevenção. J Bras Doenças Sex Transm. 2006;18(4):220-3.
49. Nadal S, Manzione CR. Vacinas Contra o Papiloma Virus Humano. Rev Bras Coloproct. 2006;26(3):337-40. DOI: http://dx.doi.org/10.1590/S0101-98802006000300017
1. Post-doctoral Student, School of Medicine, Federal University of São Paulo (Adjunct Professor, Federal University of Goiás, Pontifical Catholic University of Goiás)
2. Medical Student, School of Medicine, Pontifical Catholic University of Goiás (Medical Student, School of Medicine, Pontifical Catholic University of Goiás)
Catholic University of Goiás.
Send correspondence to:
Melissa Ameloti Gomes Avelino
Rua SB 44, Qd. 36, Lt. 24. Cond. Portal do Sol II
Goiânia - GO.Brazil. CEP: 74884-654
Paper submitted to the BJORL-SGP (Publishing Management System - Brazilian Journal of Otorhinolaryngology) on April 29, 2013.
Accepted on June 29, 2013. cod. 10891.