Year: 2013 Vol. 79 Ed. 5 - (4º)
Artigo Original
Pages: 546 to 554
Gustatory and olfactory dysfunction in laryngectomized patients
Author(s): Ada Salvetti Cavalcanti Caldas1; Vera Lúcia Dutra Facundes2; Daniele Andrade da Cunha3; Patrícia Maria Mendes Balata4; Leila Bastos Leal5; Hilton Justino da Silva6
DOI: 10.5935/1808-8694.20130099
Keywords: laryngectomy; olfaction disorders; taste disorders.
Abstract:
After total laryngectomy surgery, nasal airflow is moved permanently to the tracheostomy opening, compromising the contact of odorant molecules with the nasal cavity, which may reflect changes in the olfactory and gustatory perception in these individuals.
OBJECTIVE: To evaluate the functions of smell and taste in total laryngectomized patients. Study design: a study of series.
METHOD: The sample included a group of 25 patients submitted to total laryngectomy and another group of 25 patients who did not underwent the procedure. The taste function was evaluated by gustatory strips of filter paper. To assess the olfactory function we employed the Brief Smell Identification Test.
RESULTS: Among the laryngectomized patients there was more hypogeusia (80%, p < 0.05), as well as hyposmia (88%, p < 0.001), alone and concomitant (72%, p < 0.001). Concerning flavor discrimination, the bitter taste did not differ between the groups - which was different from the other flavors. In the olfactory aspect, laryngectomized patients performed worse in detecting warning and food-related odors. We found that a history of smoking and alcohol consumption were significantly more frequent among laryngectomized patients.
CONCLUSION: We found a decrease of gustatory and olfactory functions in total laryngectomized patients in this study.
![]()
INTRODUCTION
After total laryngectomy surgery, nasal airflow is permanently transferred to the tracheostome, compromising the arrival of odorant molecules to the nasal cavity1,2. The decrease in olfactory (hyposmia) and gustatory (hypogeusia) perceptions of individuals undergoing this intervention is often reported in the literature3,4. Currently, it is considered that the laryngectomy may cause these changes due to the interruption that occurs in the respiratory tract, as well as by changes in the epithelial structure of the nasal mucosa and in the sensorineural feedback5,6.
These sensory changes are less frequently investigated in clinical practice, since the loss of verbal communication, pulmonary complications and the psychosocial problems are evident after this surgery, and more often rehabilitated.
The present study aimed to evaluate the smell and taste perceptions in patients submitted to total laryngectomy, compared with non-laryngectomized individuals, by means of two quantitative tests.
METHOD
Study Group
We had a group of 25 patients who underwent total laryngectomy for cancer and a comparison group of 25 individuals without laryngectomy, regardless of gender and education.
The exclusion criteria for both groups were: a history of smell and taste disorders, use of medications that could impair the functions analyzed, as well as if at the time of collection the patient had rhinitis, sinusitis, and inflammatory processes in the stomatognathic system.
We had an odds-ratio of 10.22 for the group of laryngectomized individuals, our sample counted with 50 subjects, representing a 99.9% proof power, with a significance level of 0.05.
Smell Test
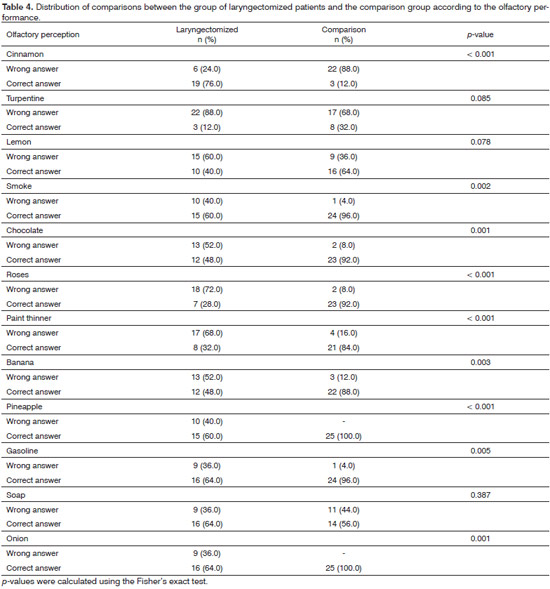
To assess the olfactory function we used The Brief Smell Identification Test - B-SIT (Sensonics Inc.®, Haddon Hts., NJ 08035) from the University of Pensilvânia7. The test consists of presenting 12 scents (cinnamon, turpentine, lemon, smoke, chocolate, roses, paint thinner, banana, pineapple, gasoline, soap, onions), contained in microcapsules of urea-formaldehyde polymers of 10-50 micrometers, fixed in strips contained in the bottom corner of 12 pages of a single booklet.
The test was of rapid administration, establishing a relative degree of olfactory function loss through percentiles.
Taste test
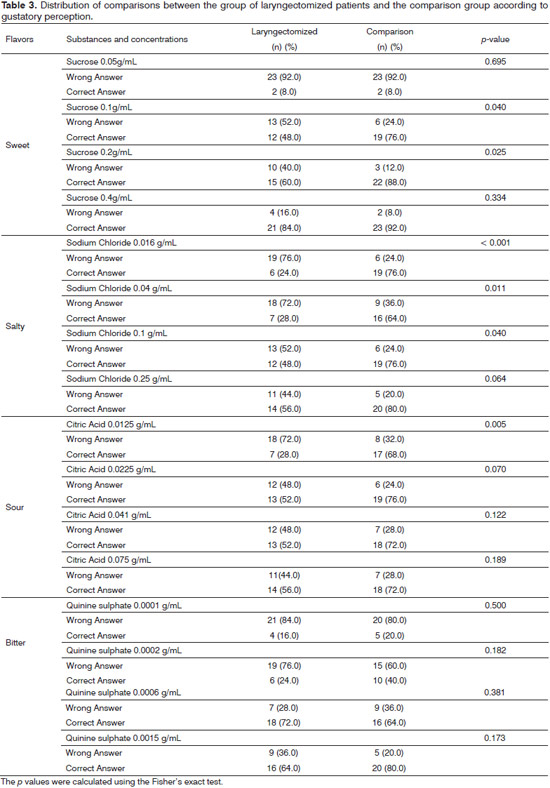
The instrument used to evaluate the gustatory function was based on the test validated by Muller et al.8. Strips of filter paper 8 cm long and 2 cm2 were impregnated with different concentrations of the following flavors: salty, sweet, bitter, sour; there were also two strips with distilled water (unflavored) used to validate the study; totaling 18 strips. We used the following concentrations: sour - 0.3 g/ml, 0.165 g/ml, 0.09 g/ml and 0.05 g/ml citric acid; bitter - 0.006 g/ml 0.0024 g/ml , 0.0009 g/mL to 0.0004 g/ml quinine sulphate; sweet - 0.4 g/ml, 0.2 g/ml, 0.1 g/ml and 0.05 g/ml sucrose; salty - 0.25 g/ml 0.1 g/ml 0.04 g/ml and 0.016 g/ml sodium chloride.
The strips were placed on the middle of the volunteer's tongue at a distance of approximately 1.5 cm from the tip of the tongue, and the test began with the lowest concentration. After evaluating each strip, the volunteer rinsed his mouth with water to remove any residue.
In accordance with recommendations in the literature,8 the taste test was conducted at least one hour after the last feeding, ingestion of any drink (except for water), having smoked or having brushed the teeth.
Statistical Analysis
The data was organized in an Excel® spreadsheet and analyzed using the SPSS version 17.0 software. For data analysis purposes we used the chi-square, Fisher's exact and ANOVA tests.
To classify the study subjects from both groups, as for their gustatory function, we used nine correct answers out of a total of 16 concentrations tested as the cutting point; classifying as hypogeusia a total less than or equal to 9; and normogeusia a total number of correct answers greater than 9. For sweet, salty and sour gustatory stimuli, the perception was classified as hypogeusia when the total number of correct answers was less than or equal to two. For the bitter gustatory stimulus, hypogeusia was considered when there was one or less correct answer8.
Proper olfactory function, according to age and gender, was classified as hyposmia when the total number of correct answers vis-à-vis the olfactory stimuli was less than nine, following the guidelines for B-SIT® application7.
Variables related to age and number of correct answers vis-à-vis the olfactory and gustatory stimuli were expressed as a mean, standard error of the mean; with respective confidence intervals at 95% level.
To compare the mean values between the groups, we employed the ANOVA test and, to compare the distributions of absolute and relative frequencies, we used the Person's chi square or Fisher's exact tests. We employed a significance level of 0.05 for all tests.
This study was approved by the Ethics in Human Research Committee, #33/2010 (CAAE: 0015.0.447.000-10) and all participants signed an Informed Consent Form (ICF).
RESULTS
Most of the laryngectomized volunteers had incomplete elementary education (21, 84.0%) (p < 0.001), a more frequent history of smoking (84%) and alcohol intake (60%) (p < 0.001) and larger number of missing teeth (p < 0.05) (Table 1).
In gustatory perception tests, the mean score of correct answers among the laryngectomized patients equaled 7.2 ± 0.48 points, ranging between 1-11 points, while in the comparison group, the mean was 10.5 ± 0.7 points, ranging from 4 to 16 points. This difference was significant (F = 15.695, p < 0.001).
Considering the cutoff point for gustatory capacity adjustment8, we noticed that the laryngectomized group had significantly more frequent hypogeusia (20; 80.0%) than the comparison group (9; 36.0%) (c2 = 5. 88, p = 0.015) (Table 2).
Hypogeusia was more frequent among laryngectomized with schooling up to middle level and a history of smoking and alcohol consumption, when compared with non-laryngectomized individuals and not all of these variables were statistically significant (Table 2).
Among the sweet, salty and sour tastes, the laryngectomized group had the most frequent percentage of identification errors, while for the bitter taste it was equal for both groups (Table 3).
In the olfactory discrimination test, the laryngectomized group had a mean level of correct answers equal to 6.0 ± 0.5, points, ranging between 3-11 points, differing significantly from the comparison group, which obtained a mean level of correct answers equal to 9.1 ± 0.3 points, ranging from 6 to 11 points (F = 31.937, p < 0.001).
Similarly to that observed on the gustatory assessment, we found that hyposmia was more frequent among males with mid-level education, as well as in the presence of a history of smoking and alcohol consumption among laryngectomized subjects and all these differences were significant (Table 2).
Considering nine points as the cutoff point for olfactory capacity adaptation, according to gender and age7, we noticed that 15 (60.0%) individuals from the comparison group had normal olfactory perception for their age and gender, compared with three (12%) from the laryngectomy group. In the comparison group, 10 (40%) subjects had abnormal olfactory discrimination for age and gender - a percentage significantly lower than the 88% in the laryngectomized group (c2 = 12.50; p < 0.001) (Table 2).
Table 4 shows the breakdown of this difference. Laryngectomized patients more often did not identify odors of smoke, chocolate, rose, turpentine, banana, pineapple, onions and gasoline, and all these differences were significant. Although the laryngectomized have presented lower scores in turpentine and lemon odor identification, differences in relation to the comparison group were not significant.
Comparing the groups regarding concomitant changes of olfactory and gustatory perception, we found that in the comparison group, normogeusia associated with normosmia was more frequent (10.40%); while in the group of laryngectomized patients there was a higher frequency of hypogeusia and hyposmia (18.72%). In cases where there was involvement of a single perception, hyposmia was more frequent than hypogeusia, especially among the laryngectomized patients (c2 = 17.74, p < 0.001).
DISCUSSION
Males, around 60 years of age, represent a worldwide prevalence of laryngeal cancer, as previously reported in the literature9-12 - having been demonstrated in this study - which showed a significant relationship between hyposmia and males among laryngectomized patients, but not hypogeusia.
Research suggests that males have the worst olfactory performance under the hypothesis that genetics and hormonal issues can be determinant for this gender difference in sensory perception13. Other researchers point out that the reduction of smell and taste is an occurrence inherent to the physiological process of aging14. These variables must also be considered in relation to the sensory losses found in this study, although hyposmia and hypogeusia are reported in the literature as frequent changes in laryngectomized patients4,15.
The low level of education found in the laryngectomized group may be associated with socioeconomic and cultural aspects; in addition, the evaluation methods used in our study required a subjective response, taking into account reports or experiences of the subject, which should be considered in understanding the results.
The interpretation of the association between hypogeusia and hyposmia and middle-level education among the laryngectomized subjects deserves caution, because it may be related to socioeconomic status and the type of occupation these patients have.
A study carried out with a standardized test to assess the olfactory function in the Brazilian population found that socioeconomic status may influence the loss of olfactory sensation. Hazardous occupational habits and exposure to pollutants are commonly portrayed in low economic status regions, which may result in olfactory deficit16. Another issue is the prevalence of this cancer in rural Brazil12, suggesting poorer living conditions and limited access to schooling.
Cigarette smoking and alcohol consumption have been referred to as the main etiological factors for the emergence of such cancer17. These harmful practices are also associated with olfactory epithelium degeneration, destruction of taste buds, and neuronal damage, which may result in hyposmia and hipogeusia18,19. These findings should be considered, since these habits were significantly more frequent in the laryngectomized participants of this study, and were associated with hypogeusia and hyposmia, it would be interesting to control these variables in subsequent studies.
Regarding the missing teeth, this variable does not seem to have influenced the aspects studied. The similarity of the groups in this item refutes the hypothesis that such variable prevails in laryngectomized patients, although age and educational level may suggest poor oral hygiene, as well as smoking and drinking20, and the very treatment of laryngeal cancer may damage the entire oral cavity.
Other researchers3,6,19,21 corroborate the hypothesis that the olfactory and taste functions are altered in patients who underwent total laryngectomy - confirmed in our results. Considering the occurrence of hyposmia and hypogeusia significantly greater in those subjects compared to subjects not submitted to laryngectomy, such evidence is still neglected in clinical practice.
The decrease in gustatory function, characterizing hypogeusia, was strongly evident in laryngectomized patients, as well as the occurrence of changes in the distribution of flavors - this features the most important contribution of this study.
Physiologically, flavor perception occurs through taste buds diffusely located on the tongue, palate, epiglottis, pharynx and larynx22,23. Sweet and bitter tastes have the same type of intracellular activation through G-protein coupled receptors, while stimulation of salty and sour tastes acts directly on specific ion channels located in the membranes of receptor cells23-25. However, in all cases, the electrical signals produced by the methods of conversion are uploaded to the central nervous system via cranial nerves VII, IX and X -responsible for the formation of synapses in specific receptor cells23,26.
It is believed that the disruption of the complex neurological connections and sensorineural feedback caused by complete removal of the larynx may account for these gustatory changes. It is important to stress that radiation therapy, often associated with the treatment of head and neck cancer, may also result in deleterious effects to sensory organs and tissues, including the oral cavity, tongue, salivary glands, the olfactory epithelium and nerves associated with the perception of smell and taste27.
Failure to observe significant changes in the bitter taste in the study groups over the other flavors, may have some explanations. The bitter flavor is part of a body protection mechanism associated with the rejection of certain foodstuffs28.
Recent studies have indicated that the bitter taste can be detected at other sites in addition to the tongue and adjacent mouth epithelium, for receptors have been found in the gastric and intestinal mucosae24,28, limiting the absorption of bitter tasting dietary toxins that escape aversion in the mouth28 .
The occurrence of hyposmia in laryngectomized individuals was confirmed in this study, with emphasis in the difficulty of perceiving certain odors. Hyposmia has been evidenced in laryngectomized patients by several authors;4,6,15 however, the mechanisms responsible for the decrease in the olfactory function of laryngectomized patients are not yet fully understood. The definitive change in nasal airflow to the tracheostome has been the most cited theory by researchers as the cause of this sensory dysfunction, given that smell depends on chemoreceptors located in the olfactory epithelium to respond to the presence of molecules in the air4,6.
Other authors have demonstrated theories related to degenerative diseases of the olfactory epithelium5,21 as well as damage caused to the complex sensorineural mechanisms due to the total laryngectomy surgery29.
The inability to detect smoke odors and other smell-related signs of danger in laryngectomized patients has been reported in studies, and this may jeopardize the safety of these individuals,3,19 similar to the results presented here, in which the identification of "warning odors" was significantly decreased.
The difficulty in the perception of certain odors can influence even food-related pleasures, resulting in weight loss and contributing to malnutrition19. Likewise, the loss of the perception of bodily odors may result in socializing difficulties3. Our findings suggest that these problems can cause feeding problems and therefore nutritional issues in this population, requiring further investigations in future studies.
Paint thinner -a common solvent in North America - smell interpretation proved unfamiliar to the participants in this study, which may be related to cultural characteristics, and therefore, without significant differences in the results presented here.
Studies detail the association between the sensory functions of smell and taste as responsible for defining the taste of food, closely related to eating habits, nutritional aspects and pleasure during eating30,31. It is important to report the significant relationship between the smell and taste functions identified here, with evidence of concomitant hypogeusia and hyposmia in more than half of the total laryngectomized group.
Nasal airflow interruption in the total laryngectomized may not only negatively affect olfactory perception but also their gustatory identification skills, as pointed out in our results. During the olfactory test, we observed facial muscle movements in laryngectomized individuals, suggesting an attempt to force nasal aeration for the consequent stimulation of nasal olfactory epithelium. While not a variable considered in our study, it is important to note that, during chewing, there is the induction of an air stream into the oral cavity by which the olfactory organ is stimulated through the nasopharynx3,23. When food approaches the mouth during the respiratory mechanism of inspiration, the olfactory epithelium is also stimulated, thus acting in conjunction with other sensory functions to determine the food flavor31-33.
The international literature4,6,21 has stressed the importance of giving greater emphasis to the treatment of these sensory functions; however, it is limited to only reporting as to the decline of these functions without qualifying with regards to the impaired distribution of tastes and odors.
This study fills this knowledge gap, proving that the perception of salty, sweet and bitter flavors are impaired in laryngectomized individuals, as well as the identification of important warning odors and other smells directly related to feeding, also reduced in this population. Thus, interventions seeking to stimulate the limitations of these sensory functions should be part of the therapeutic scope aimed at individuals submitted to total laryngectomy.
In clinical practice, it is relevant to evaluate these sensory functions and to develop rehabilitation programs for this population, given that the smell and taste alterations may trigger changes in eating habits and impact the pleasure associated with this activity and on the nutritional status of these subjects, in addition to reducing the alert in risky situations, compromising the quality of life of these individuals.
CONCLUSION
The decrease in gustatory and olfactory functions in laryngectomized individuals was evidenced in this study. In discriminating flavors, the bitter taste was not different between the groups at the expense of other flavors. In the olfactory aspect, the laryngectomized individuals performed worse in detecting warning and food-related odors. It is assumed that the decrease in olfactory function may be directly related to the decrease in the gustatory perception, considering the association between these functions for defining food taste as well as concurrent hypogeusia and hyposmia in laryngectomized individuals.
REFERENCES
1. Hannickel S, Zago MMF, Barbeira CBS, Sawada NO. O comportamento dos laringectomizados frente à imagem corporal. Rev Bras Cancerol. 2002;48(3):333-9.
2. Barbosa LNF, Santos DA, Amaral MXF, Gonçalves AJ, Bruscato WL. Repercussões psicossociais em pacientes submetidos a laringectomia total por câncer de laringe: um estudo clínico-qualitativo. Rev SBPH. 2004;7(1):45-58.
3. van Dam FS, Hilgers FJ, Emsbroek G, Touw FI, van As CJ, de Jong N. Deterioration of olfaction and gustation as a consequence of total laryngectomy. Laryngoscope. 1999;109(7 Pt 1):1150-5.
4. Ward E, Coleman A, van As-Brooks C, Kerle S. Rehabilitation of olfaction post-laryngectomy: a randomised control trial comparing clinician assisted versus a home practice approach. Clin Otolaryngol. 2010;35(1):39-45. DOI: http://dx.doi.org/10.1111/j.1749-4486.2009.02050.x
5. Miani C, Ortolani F, Bracale AM, Petrelli L, Staffieri A, Marchini M. Olfactory mucosa histological findings in laryngectomees. Eur Arch Otorhinolaryngol. 2003;260(10):529-35. PMID: 12835945 DOI: http://dx.doi.org/10.1007/s00405-003-0638-3
6. Risberg-Berlin B, Rydén A, Möller RY, Finizia C. Effects of total laryngectomy on olfactory function, health-related quality of life, and communication: a 3-year follow-up study. BMC Ear Nose Throat Disord. 2009;9:8.
7. Doty RL. The Brief Smell Identification Test TM Administration Manual. New Jersey: Sensonics Inc; 2001. p.2-10.
8. Mueller C, Kallert S, Renner B, Stiassny K, Temmel AF, Hummel T, et al. Quantitative assessment of gustatory function in a clinical context using impregnated "taste strips". Rhinology. 2003;41(1):2-6.
9. Oliveira Junior FJM, Cesse EAP. Morbimortalidade do câncer na cidade do Recife na década de 90. Rev Bras Cancerol. 2005;51(3):201-8.
10. Wünsch Filho V. The epidemiology of laryngeal cancer in Brazil. São Paulo Med J. 2004;122(5):188-94. PMID: 15558139 DOI: http://dx.doi.org/10.1590/S1516-31802004000500002
11. Brasil. Ministério da Saúde. Instituto Nacional de Câncer-INCA. Câncer. Rio de Janeiro: INCA; 2011. PMID: 21079888
12. Pernambuco Lde A, Vilela MB. Larynx cancer mortality in the State of Pernambuco - Brazil - 2000-2004. Braz J Otorhinolaryngol. 2009;75(2):222-7.
13. Doty RL, Cameron EL. Sex differences and reproductive hormone influences on human odor perception. Physiol Behav. 2009;97(2):213-28. DOI: http://dx.doi.org/10.1016/j.physbeh.2009.02.032
14. Boyce JM, Shone GR. Effects of ageing on smell and taste. Postgrad Med J. 2006;82(966):239-41. PMID: 16597809 DOI: http://dx.doi.org/10.1136/pgmj.2005.039453
15. Morales-Puebla JM, Morales-Puebla AF, Jiménez-Antolín JA, Muñoz-Platón E, Padilla-Parrado M, Chacón-Martínez J. Olfactory rehabilitation after total laryngectomy. Acta Otorrinolaringol Esp. 2010;61(2):128-34. DOI: http://dx.doi.org/10.1016/j.otorri.2009.10.011
16. Silveira-Moriyama L, Azevedo AM, Ranvaud R, Barbosa ER, Doty RL, Lees AJ. Applying a new version of the Brazilian-Portuguese UPSIT smell test in Brazil. Arq Neuropsiquiatr. 2010;68(5):700-5. PMID: 21049178 DOI: http://dx.doi.org/10.1590/S0004-282X2010000500005
17. Sartor SG, Eluf-Neto J, Travier N, Filho VW, Arcuri ASA, Kowalski LP, et al. Riscos ocupacionais para o câncer de laringe: um estudo caso-controle. Cad Saúde Pública. 2007;23(6):1473-81. DOI: http://dx.doi.org/10.1590/S0102-311X2007000600022
18. Rupp CI, Fleischhacker WW, Hausmann A, Mair D, Hinterhuber H, Kurz M. Olfactory functioning in patients with alcohol dependence: impairments in odor judgements. Alcohol Alcohol. 2004;39(6):514-9. PMID: 15456691 DOI: http://dx.doi.org/10.1093/alcalc/agh100
19. Leon EA, Catalanotto FA, Werning JW. Retronasal and orthonasal olfactory ability after laryngectomy. Arch Otolaryngol Head Neck Surg. 2007;133(1):32-6. PMID: 17224519 DOI: http://dx.doi.org/10.1001/archotol.133.1.32
20. Shinkai RSA, Del Bel Cury AA. O Papel da Odontologia na equipe interdisciplinar: contribuindo para a atenção integral ao idoso. Cad Saúde Pública. 2000;16(4):1099-9. DOI: http://dx.doi.org/10.1590/S0102-311X2000000400028
21. Fujii M, Fukazawa K, Hatta C, Yasuno H, Sakagami M. Olfactory acuity after total laryngectomy. Chem Senses. 2002;27(2):117-21. PMID: 11839609 DOI: http://dx.doi.org/10.1093/chemse/27.2.117
22. Paula RS, Colares FCJ, Toledo JO, Nóbrega OT. Alterações gustativas no envelhecimento. Rev Kairós. 2008;11(1):217-35.
23. Farmer MN, Raddin RS, Roberts JD. The relationship between taste, olfaction, and nutrition in the cancer population. J Support Oncol. 2009;7(2):70-2.
24. Behrens M, Meyerhof W. Gustatory and extragustatory functions of mammalian taste receptors. Physiol Behav. 2011;105(1):4-13 PMID: 21324331
25. Niki M, Yoshida R, Takai S, Ninomiya Y. Gustatory signaling in the periphery: detection, transmission, and modulation of taste information. Biol Pharm Bull. 2010;33(11):1772-7. DOI: http://dx.doi.org/10.1248/bpb.33.1772
26. Costa MM, Santana E, Almeida Jd. Oral taste recognition in health volunteers Arq Gastroenterol. 2010;47(2):152-8.
27. Hong JH, Omur-Ozbek P, Stanek BT, Dietrich AM, Duncan SE, Lee YW, et al. Taste and odor abnormalities in cancer patients. J Support Oncol. 2009;7(2):58-65.
28. Jeon TI, Seo YK, Osborne TF. Gut bitter taste receptor signalling induces ABCB1 through a mechanism involving CCK. Biochem J. 2011;438(1):33-7.
29. Henkin RI, Larson AL. On the mechanism of hyposmia following laryngectomy in man. Laryngoscope. 1972;82(5):836-43. DOI: http://dx.doi.org/10.1288/00005537-197205000-00010
30. Hilgers FJ, van Dam FS, Keyzers S, Koster MN, van As CJ, Muller MJ. Rehabilitation of olfaction after laryngectomy by means of a nasal airflow-inducing maneuver: the "polite yawning" technique. Arch Otolaryngol Head Neck Surg. 2000;126(6):726-32. DOI: http://dx.doi.org/10.1001/archotol.126.6.726
31. Leopold D, Holbrook EH, Noell CA. Disorders of Taste and Smell. eMedicine Medscape. 2009 [Acesso 27 set. 2011]. Disponível em: http://emedicine.medscape.com/article/861242-overview
32. Leow LP, Huckabee ML, Sharma S, Tooley TP. The influence of taste on swallowing apnea, oral preparation time, and duration and amplitude of submental muscle contraction. Chem Senses. 2007;32(2):119-28. DOI: http://dx.doi.org/10.1093/chemse/bjl037
33. Smith DV, Margolskee RF. Making sense of taste. Sci Am. 2001;284(3):32-9. PMID: 11234504 DOI: http://dx.doi.org/10.1038/scientificamerican0301-32
1. MSc. - Occupational Therapist
2. PhD in Neuropsychiatry and Behavioral sciences; Associate Professor - Department of Occupational Therapy - Federal University of Pernambuco
3. PhD in nutrition; Substitute Professor - Department of Speech Pathology - Federal University of Pernambuco
4. PhD in Neuropsychiatry and Behavioral sciences; Speech and Hearing Therapist
5. PhD in Pharmaceutical Sciences; Associate Professor - Department of Pharmacy - Federal University of Pernambuco
6. PhD in Nutrition; Associate Professor - Department of Speech Therapy - Federal University of Pernambuco. MSc. in Pathology - Federal University of Pernambuco - UFPE - Recife (PE) - Brazil.
Send correspondence to:
Ada Salvetti Cavalcanti Caldas
Rua Guedes Pereira, nº 180, apto. 903. Parnamirim
Recife - PE. Brazil. CEP: 52060-150
Paper submitted to the BJORL-SGP (Publishing Management System - Brazilian Journal of Otorhinolaryngology) on March 19, 2013.
And accepted on June 29, 2013. cod. 10818.