Year: 2013 Vol. 79 Ed. 3 - (20º)
Artigo de Revisão
Pages: 391 to 400
Brazilian Academy of Rhinology position paper on topical intranasal therapy
Author(s): João Ferreira de Mello Júnior1; Olavo de Godoy Mion2; Nilvano Alves de Andrade3; Wilma Terezinha Anselmo-Lima4; Aldo Eden Cassol Stamm5; Washingthon Luiz de Cerqueira Almeida3; Pedro Oliveira Cavalcante Filho6; Jair de Carvalho e Castro7; Francini Grecco de Melo Padua8; Fabrizio Ricci Romano9; Rodrigo de Paulo Santos3; Renato Roitmann10; Richard Louis Voegels1; Roberto Campos Meirelles11; Leonardo Conrado Barbosa Sá12; Moacyr Tabasnik Moacyr13; Marco Cesar Jorge dos Santos14; Roberto Eustáquio Santos Guimarães15
DOI: 10.5935/1808-8694.20130067
Keywords: medication systems; nasal cavity;nose; therapeutics.
Abstract:
This documents aims at educating those who treat sinonasal diseases - both general practitioners and specialists - about topical nasal treatments. By means of scientific evidence reviews, the Brazilian Academy of Rhinology provides its practical and updated guidelines on the most utilized topical nasal medication, except for the drugs that have topical antibiotics in their formulas.
![]()
INTRODUCTION
Inflammatory diseases of the nose and paranasal sinuses are among the most prevalent conditions to affect the general population. Diseases such as allergic and non-allergic rhinitis, acute and chronic rhinosinusitis with and without nasal polyps, cause significant decrease in the quality of life of the affected patients, and adversely affect one's ability to perform activities connected to work, leisure, and socialization. These patients require specific specialized care.
Nasal topical medications are extremely important in the care of patients with inflammatory diseases of the nose and paranasal sinuses and upper airway infections. Although some drug classes have been used for decades, new medications have been made available to patients.
Given the prevalence of these diseases, significant direct and indirect expenditures are associated with treatment, particularly when long term therapies are considered. The costs associated with treatment should not be neglected. Patients, their families, the public health care system and society may experience significant savings as a result of the judicious use of medication.
This paper aims to provide clarification to specialist physicians and general practitioners on the treatment of nose and sinus diseases with topical nasal medication. The Brazilian Academy of Rhinology reviewed the scientific evidence available to offer a practical updated overview on the most commonly used non-antibiotic topical nasal medications.
Topical intranasal steroids
Intranasal steroids have been effectively used to treat allergic rhinitis, rhinosinusitis, and nasal polyps1, in addition to a wide array of non-allergic rhinitis, such as idiopathic, vasomotor, and gestational rhinitis2.
Increased knowledge on the pharmacology of glucocorticoid and steroid receptors has enabled the development of molecules designed to potently reach specific localized activity with minimum risk of systemic side effects3.
The introduction of topical glucocorticoids and steroids (GCS) has significantly enhanced the treatment of upper and lower airway diseases. Their clinical efficacy may depend partly on the ability to reduce eosinophilic function and infiltration by inhibiting the activation and viability of eosinophils4-6. They may also act to reduce the release of chemotactic enzymes on the nasal mucosa and polyp epithelial cells7. The potency of these drugs is lesser in nasal polyps than in the nasal mucosa, thus suggesting that nose polyps may present induced inflammatory resistance to therapy with steroids8.
The biological effect of GCS is mediated by the intracellular activation of glucocorticoid receptors9 expressed in most tissues and cells. Two different GCS receptor isoforms have been identified in humans, alpha (GRa) and beta (GRb), both originated in the same gene and divided after the primary transcription of the glucocorticoid receptor10. As GRa binds to a hormone, it increases pro-inflammatory genetic transcription and produces most of the anti-inflammatory effect seen in GCS through protein interactions between glucoreceptors and transcription factors such as AP-1 and NF-KB. GRb does not bind to steroids, but may interfere with GRa function. A number of mechanisms are probably involved in resistance to the anti-inflammatory effect provided by GCS, including exaggerated expression of GRb or reduced expression of GRa. Increases in GRb expression have been seen in patients with nasal polyps11,12, while downregulation of GRa levels has been reported after treatment with GCS13,14 as one of the possible explanations for secondary resistance to GCS15. Theoretically, the anti-inflammatory effects of GCS may be seen in allergic and non-allergic rhinosinusitis, for instance in cases of infectious rhinosinusitis; tissue eosinophilia is also found in patients with chronic or persistent rhinosinusitis16.
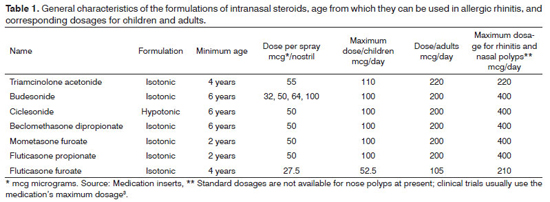
Each glucocorticoid and steroid has unique molecular, pharmacokinetic, and pharmacodynamic properties, which lead to drugs with different modes of action. For example, furoate increases GCS potency and selectivity for the mineralocorticoid receptor17. Ciclesonide is an inactive drug converted to a pharmacologically active metabolite, des-isobutyryl-ciclesonide, by upper and lower airway esterases18. Ciclesonide and budesonide - the latter not a prodrug - also form fatty acid esters after topical administration on the nasal mucosa (budesonide oleate and budesonide palmitate), thus contributing to their intracellular retention in the nasal mucosa19. Pharmacological studies on potency using affinity as the only criterion yielded a classification for GCS. Mometasone furoate, fluticasone furoate, and fluticasone propionate were ranked as the most potent intranasal glucocorticoids and steroids20. The side chains in furoate and propionate allow these esters to be highly lipophilic, a quality that may facilitate their absorption by the nasal mucosa and further progression through the cell membrane phospholipase. These compounds do not present significant systemic absorption, with values under 1%, together with ciclesonide21.
Studies have shown higher rates of systemic absorption in older GCS, such as beclomethasone dipropionate, triamcinolone acetonide, and budesonide, with bioavailability ranging between 34% and 49%. However, the one-year studies carried out on mometasone, fluticasone propionate, and budesonide to assess the potential systemic side effects in children did not reveal adverse effects on the hypothalamic-pituitary-adrenal axis or upon its growth. In theory, medications with lower levels of bioavailability should be given preference for being closer to the pharmacokinetic and pharmacodynamic traits desired in an ideal course of therapy. However, studies have failed to confirm this assumption22-26. These drugs are very useful in managing symptoms and, therefore, can be administered in a chronic regimen (Table 1).
Topical intranasal glucocorticoids and steroids are effective against seasonal and perennial allergic rhinitis, non-allergic rhinitis, and episodic rhinitis, as they effectively manage all rhinitis symptoms, including nasal congestion.
Onset of action is usually slower than oral and intranasal antihistamines, occurring within 12 hours in most patients or in three to four hours in some subjects after provocation tests. Full effect takes longer to occur.
When compared to other drug classes, topical GCS are more effective than the combination of oral antihistamines and antileukotriene agents for seasonal or perennial allergic rhinitis. Their efficacy is comparable to that of oral antihistamines for ocular symptoms of allergic rhinitis. Fluticasone furoate has been the most frequently studied drug for ocular symptoms of patients with rhinoconjunctivitis, and was found to be significantly more effective than placebo and just as effective as antihistamines27,28.
No systemic side effects have been observed in adults, nor have adverse impact on the growth of children with perennial allergic rhinitis been described, when the drug is administered in the recommended dosages. Local side effects are minimal, but nasal irritation and bleeding may occur. Cases of septal perforation have been reported, although rarely. Drug effect in children and pregnant women is very similar to that observed in adult subjects. However, given the potential unexpected consequences, GCS must be judiciously prescribed and administered to these two populations.
Studies on the use of topical intranasal GCS in children have not described adverse effects or significant levels of systemic absorption. In pregnant women there is always the concern with the embryo and the association with cleft palate, but up to now no teratogenic effects have been reported, despite the few studies done on the matter. Therefore, the risks and benefits provided by these medications must be considered when they are prescribed to pregnant individuals. The only intranasal GCS assigned category B by the Food and Drug Administration for use in pregnant subjects is budesonide29. Additionally, the recommendation states that it be used in the lowest dosage possible and for as short as possible. When glaucoma is considered, the literature features reports associating intraocular pressure worsening and use of intranasal GCS. However, other studies have not correlated onset of glaucoma and chronic use of mometasone and ciclesonide. Patients with glaucoma prescribed topical intranasal GCS should be followed by an ophthalmologist until more knowledge on the topic has been gathered.
Clinical trials indicate that there is no difference in efficacy between intranasal GCS medications, but most have compared them to placebo. Few studies have made head-to-head comparisons between GCS medications to assess efficacy.
Treatment of rhinosinusitis with topical GCS
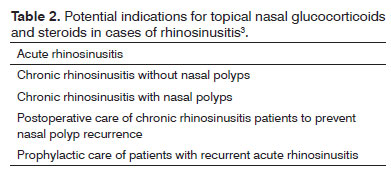
Many are the indications to use topical intranasal GCS in rhinosinusitis, ranging from acute to chronic disease (Table 2).
Considering rhinosinusitis, many studies have looked into single-drug therapy regimens with GCS and GCS as adjuvant drugs. Authors have studied budesonide30-38, mometasone39,40, fluticasone furoate, fluticasone propionate41-47, and beclomethasone dipropionate48-52 and reported improved symptom scores in regimens with antibiotics and significant differences when other criteria were analyzed, such as x-ray images and CT scans, or nasal peak flows and acoustic rhinometry or rhinomanometry. One study reported significant reductions in symptoms of acute rhinosinusitis comparing placebo and antibiotics53.
Topical GCS medications have known effects upon nasal polyps and associated symptoms such as obstruction, secretion, and sneezing, although smell is affected to a lesser degree. High level evidence is available on polyp size reduction.
In regards to prevention, low level evidence is available on the prophylactic effects of nasal GCS upon recurrent acute rhinosinusitis.
Scientific knowledge on GCS, their effects on the glucocorticoid receptor and cell transcription processes has grown and improved our understanding of this drug class and its use in therapeutic settings.
Despite the significant amount of information available, the clinical differences between each compound are still not clear. As a drug class, intranasal GCS medications have comparable levels of efficacy in the treatment of upper airway inflammatory diseases.
The commercially available GCS medications are close to reaching the ideal pharmacokinetic and pharmacodynamic properties of this drug class in topical nasal applications, namely:1. High affinity to the receptor, potency, and specificity to the nasal mucosa;However, studies are being carried out to find even better drugs considering the criteria above.
2. Low systemic bioavailability;
3. High rate of hepatic clearance and fast systemic elimination;
4. Single daily dosage.
Topical nasal antihistamines
Intranasal antihistamines may be considered as the first-line treatment for allergic and non-allergic non-infectious rhinitis54,55, and are as effective or better than second generation oral antihistamines in the treatment of seasonal allergic rhinitis56,57, although they are usually less effective than intranasal GCS in the treatment of allergic rhinitis58. However, they have been associated with clinically significant effect upon nasal congestion59.
The only intranasal antihistamine currently available in our practice is azelastine, a drug characterized by good efficacy and quick onset of action60. Azelastine has been approved for the treatment of seasonal and perennial allergic rhinitis, and showed effect upon nasal congestion, rhinorrhea, sneezing, and nasal itching. It was the first antihistamine associated with significant clinical reduction of nasal congestion61, in addition to being the first effectively used in cases of non-allergic rhinitis. However, given their systemic absorption, intranasal antihistamines have been associated with sedation and may inhibit the histamine reaction in skin tests1.
Several studies have reported that their efficacy against seasonal rhinitis is greater than or equal to that of second generation oral antihistamines59. A systematic review encompassing nine randomized controlled trials comparing intranasal antihistamines and intranasal GCS62 concluded that intranasal GCS medications are more effective in managing the nasal symptoms of perennial and seasonal rhinitis. Significant benefits may be yielded when intranasal antihistamines and intranasal GCS are combined55, although such combination is not commonly utilized.
Azelastine is formulated as an aqueous solution and administered in the form of a nasal spray with a dosage of 1 mg/ml. It is recommended that each nostril be sprayed twice a day in patients above 12 years of age. Half the dosage is recommended for children aged five and older. Onset of action takes about 15 minutes for significant clinical improvement, a fact that places azelastine as a drug to be used in the early stages of allergy bouts1.
Clinical trials on azelastine reported that approximately 19% of the patients complained of bitter taste and 11% reported sleepiness1.
Intranasal antihistamines are absorbed by the gastrointestinal tract, and azelastine may thus suppress reactions in the skin test for up to 48 hours63.
Intranasal disodium cromoglycate
Intranasal disodium cromoglycate has been effective for some patients in the prevention and treatment of allergic rhinitis, and has not been associated with side effects. It prevents immediate allergic reactions more than it provides symptom relief after the reaction has begun64. It is also used in maintenance allergic rhinitis treatment, with onset of action ranging from four to seven days. Full action may take weeks to occur65. In episodic rhinitis, use immediately before exposure to antigens protects subjects for four to eight hours against immediate allergic response66.
It acts by inhibiting the degranulation of mast cells, consequently preventing the release of immediate allergic response and allergic inflammation mediators. This drug has a unique mode of action and is known for not being a bronchodilator, antihistamine, or direct anti-inflammatory agent67.
The 4% spray solution is indicated for the treatment of seasonal and perennial allergic rhinitis. When used in the treatment of symptoms of seasonal allergic rhinitis, cromoglycate must be started early on in the beginning of the allergy season. Effect is usually noted four to seven days after the start of treatment. However, more severe or perennial cases require two weeks or more until maximum effect sets in. Very symptomatic patients may need combined therapy with antihistamines and/or nasal decongestants during the first days of treatment, given the need of proper contact with the nasal mucosa for the drug to be effective1. Treatment must be continued by offering the patient a maintenance dosage that is effective for the remainder of the season or period of exposure to the antigens.
Cromoglycate has been effectively used in the treatment of episodic rhinitis when contact with or exposure to the allergen can be anticipated. In these cases, its onset of action appears to be faster. The protective effect provided by cromoglycate against antigen nasal provocation persists for four to eight hours after administration, thus enabling preventive care when exposure can be predicted, as in the case of veterinarians with allergies.
In controlled trials, cromoglycate was better than placebo. A double-blind randomized placebo-controlled trial with children aged between two and five years of age revealed cromoglycate provided relief from allergic rhinitis symptoms. However, cromoglycate was in general less effective than intranasal GCS, and has not been properly studied in relation to leukotriene antagonists and antihistamines68-70.
Cromoglycate is a safe medication, Adverse effects are usually mild and local, and include sneezing and sensation of burning. No cases of crust of septal perforation have been described. There is no clinical evidence indicating patients could experience overdosages of cromoglycate.
Given its excellent safety profile and absence of significant interactions with other drugs, cromoglycate may be considered and prescribed to young children and pregnant women71,72, thus serving as a valuable alternative when other nasal sprays are contraindicated or not tolerated by the patient.
Proper patient selection is critical when cromoglycate is considered. Reviews have described the limited role cromoglycate has in the treatment and prevention of allergic rhinitis symptoms71. There is no evidence that cromoglycate may benefit patients with non-allergic non-infectious rhinitis (NARES) or nasal polyps72.
Nasal decongestants
Nasal congestion is one of the most troubling symptoms for patients with rhinitis73. The medications with the best effect on this symptom are nasal decongestants, sympathomimetic drugs that act directly on the capacitance vessels of the turbinates.
They are available in oral (pseudoephedrine and phenylephrine) and intranasal topical (phenylephrine, naphazoline, and oxymetazoline) formulations. Both can produce increased blood pressure, agitation, headaches, anxiety, insomnia, tremor, palpitations, dry mucosa, urinary retention in patients with enlarged prostates, glaucoma worsening, and thyrotoxicosis2,74.
Topical vasoconstrictors usually start acting within around 10 minutes. However, when used for more than five or ten days, they may lead to the appearance of drug-induced rhinitis as a consequence of their rebound effect. The ARIA (Allergic Rhinitis and its Impact on Asthma) initiative does not recommend its use on children with allergic rhinitis, and states that adults should limit its use to a maximum of five days2.
These drugs must be judiciously used in patients taking monoamine oxidase inhibitors (MAOIs), antihypertensive drugs, digitalis, and L-DOPA.
Inert cellulose powder
Inert cellulose powder has been used as a thickener in various liquid formulations of nasal application. Natural inert cellulose was recently approved for use in rhinitis patients in Brazil.
Cellulose powder is known to hamper bacterial growth, even though its exact mode of action in allergic rhinitis has not been fully explained. It is believed to form a gelatinous membrane on the epithelium when applied to the nasal mucosa. The gelatinous membrane has greater surface tension than mucus, and acts as a more effective barrier to antigen penetration, which cannot reach the effector cells. Thus, it would not affect symptoms, but the entire allergic process by impacting the development of the allergic inflammatory cascade75.
A study carried out on the effects of inert cellulose powder in allergic rhinitis by pollen showed that patients using micronized cellulose powder required significantly less salvage medication to manage nasal symptoms76.
Additionally, patients with allergic rhinitis by mites using cellulose powder had fewer nasal symptoms in nasal provocation tests for these antigens77.
As it is not a medication, the medical literature on inert cellulose powder is scarce and unclear about its mode of action, action on the mucociliary barrier, and dosage.
Saline solutions
The use of saline solutions in nasal hygiene has been recommended by specialists, but their effects may go beyond those of an adjuvant therapy1-3. Saline solution has been indicated for patients with allergic rhinitis, non-allergic rhinitis, acute and chronic rhinosinusitis, and even non-specific conditions such as postnasal drip1-3.
Our patients have been advised for years to use isotonic (0.9%) sodium chloride solutions. In the 1990's, hypertonic (2% or 3%) sodium chloride solutions were introduced. They were initially used in nose surgery postoperative care to ease the removal of crusts. More recently, the medical literature has reported that these solutions are also useful in controlling nasal symptoms of patients with other clinical conditions such as rhinitis.
Nasal washing with isotonic saline solution is an easy, well tolerated and beneficial procedure with practically no relevant adverse side effects78. The most common complaint associated with it is sensation of local irritation, in some cases related to the concentration or conservatives added to the solution.
Benzalkonium chloride is a surfactant belonging to the quaternary ammonium group used to prevent contamination by bacteria and preserve pharmacological activity of topical nasal, ocular, auricular, and cutaneous medication. Its hydrophobic and cationic groups act to increase the permeability of bacterial cell membrane, thus conferring it bactericidal properties79,80.
In vitro studies have shown that the deleterious effects of benzalkonium chloride on ciliary beat rates decreases with drops in pH. This finding has been observed in pH reductions to 7.4 and even 6.0. As the nasal mucus pH is situated between 5.5 and 6.0, it is reasonable to assume that this fact could interfere with the direct correspondence between in vitro results and physiological conditions of the nasal cavities. There is no concrete evidence to state that benzalkonium chloride hurts the human mucociliary barrier in vivo, but the substance has been accounted for the bitter taste of certain nasal preparations81,82.
Nasal washing with hypertonic solution (3%) is safe and carries minimal side effects. A few cases of local irritation, itching, burning, otalgia, and sensation of pressure on the face have been described in the literature83.
The exact mode of action of nasal washing with saline solution is still being studied. Some possibilities have been considered, such as mechanical cleaning of the nose, dilution of nasal mucus, induction of rhinorrhea, and effects upon ciliary beat rate and ciliary clearance83.
The use of saline solution increases the movements of mucus toward the rhinopharynx by mechanical action. The justification for this hypothesis is based on patient reports of increased efficacy in forced washing cycles, such as in cases in which saline solution is gently injected into the nasal cavity with a syringe. Additionally, when forced washing is performed, crusts are softened and removed more easily. Thick secretion can also become less viscous and be more easily removed84.
In nasal diseases, various chemical inflammatory mediators are released and dissolved in mucus, acting directly and indirectly on the mucosa and inducing edema and ciliary beat alterations. Saline solution removes and/or dilutes these mediators, thus reducing local inflammation and, consequently, edema. A reduction on levels of histamine and leukotriene C4 has been observed in the nasal washes of patients using hypertonic saline solution85. Additionally, the use of hypertonic solution on the nasal mucosa may induce the release of substance P, an important neuropeptide that induces rhinorrhea in a dose-dependent fashion86.
Ciliary beating is a relevant defense mechanism of the respiratory tract, and reductions in it have been associated with respiratory diseases such as rhinitis, rhinosinusitis, asthma, and otitis media. In healthy individuals without nasal complaints, the saccharin test (in vivo) has shown that hypertonic saline solution (3%) significantly improves ciliary beating, an event not seen with isotonic saline solution87.
In vitro studies have shown that both isotonic and hypertonic solutions may affect ciliary beat rates. In vitro ciliated cells of the nasal mucosa treated with saline solutions in different concentrations (0.06%; 0.12%; 0.9%; 3.0%; 7.0%) only presented altered ciliary beating in hypertonic concentrations88. On the other hand, some authors have observed moderate negative impact on ciliary beating in cells treated with isotonic saline solution. When solutions in higher concentrations were used (7%; 14%), ciliary beating ceased after five minutes, a finding reversible only in 7% saline solution89.
An in vitro study - thus in the absence of the protection offered by nasal mucus - on cultured human nasal epithelial cells compared the effects of hypotonic (0.3%), isotonic, and hypertonic (3.0%) saline solutions on gland secretion and cell morphology. The three solutions did not affect total mucin secretion. However, in the 0.3% and 3.0% concentrations the epithelium was injured, a condition not observed with isotonic saline solution90.
Another interesting point concerning nasal washing refers to the pH of the used solution. A double-blind randomized trial found that buffered (pH = 8) and non-buffered hypertonic saline solutions significantly increased mucociliary clearance, without significant differences between the two solutions91.
Tonicity of saline solutions was found to affect the absorption of topical medications. The bioavailability of calcitonin applied on the nose in hypotonic or hypertonic saline solutions was four to five times greater than when an isotonic formulation was used92. Thus, the cell concentration of ciclesonide is higher when nasal topical ciclesonide is applied with hypotonic saline solution versus isotonic saline solution93.
Saline solutions can be applied in three different ways. The simplest involves the use of negative pressure, in which saline solution is poured on the palm of the hand and introduced in the nasal fossae by forced inhalation. Another method employs positive pressure to distribute the fluid in the nasal fossae, such as when syringes or eyedroppers are used. The last option is to use sprays, an easy-to-carry option for patient medication94. Each of the possibilities has its pros and cons, but patient compliance must be considered in any of them.
CT scans done after nasal washing with saline solution and ionic contrast have shown greater penetration of the solution into the maxillary and ethmoid sinuses when positive pressure approaches were used than when sprays were employed94. Likewise, an analysis of airflow through the different areas of the nasal fossae showed that airflow was very limited in the paranasal sinuses, which are protected by the uncinate process and the middle nasal concha, thus explaining the reduced effectiveness of sprays in reaching these sites95.
It is worth mentioning that these approaches refer to the application of saline solution, and not nasal spray medication. For these, it is recommended that patients gently blow their noses before using the topical medication to facilitate penetration. The goal is that the medication makes it to the turbinates, and thus patients have to be instructed to direct the tip of the delivery device to the lateral wall of the nasal cavity21. In practical terms, patients are advised to apply the medication on the left nasal fossa with the right hand and vice-versa. As the patient applies pressure onto the device's valve, it is recommended that he/she closes the contralateral nostril with a finger while air is gently inhaled during the application of the medication96.
Although nasal hygiene with saline solution is recommended by a number of consensus papers, the literature on the topic is limited. More clinical and laboratory trials are required. Nonetheless, some points must be observed, such as not using cold or hot solutions. Saline solution does not ease symptoms promptly, and patient compliance may become an issue. However, the qualities of saline solution (low cost, few adverse side effects, etc) fully justify its use.
REFERENCES
1. Wallace DV, Dykewicz MS, Bernstein DI, Blessing-Moore J, Cox L, Khan DA, et al.; Joint Task Force on Practice; American Academy of Allergy; Asthma & Immunology; American College of Allergy; Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122(2 Suppl):S1-84. http://dx.doi.org/10.1016/j.jaci.2008.06.003 PMid:18662584
2. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al.; World Health Organization; GA(2)LEN; AllerGen. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63 Suppl 86:8-160. http://dx.doi.org/10.1111/j.13989995.2007.01620.x PMid:18331513
3. Fokkens W, Lund V, Mullol J.; European Position Paper on Rhinosinusitis and Nasal Polyps group. European position paper on rhinosinusitis and nasal polyps 2007. Rhinol Suppl. 2007;(20):1-136. PMid:17844873
4. Mullol J, Xaubet A, López E, Roca-Ferrer J, Picado C. Comparative study of the effects of different glucocorticosteroids on eosinophil survival primed by cultured epithelial cell supernatants obtained from nasal mucosa and nasal polyps. Thorax. 1995;50(3):270-4. http://dx.doi.org/10.1136/thx.50.3.270 PMid:7660341 PMCid:1021191
5. Mullol J, Xaubet A, López E, Roca-Ferrer J, Carrión T, Roselló-Catafau J, et al. Effects of topical anti-inflammatory drugs on eosinophil survival primed by epithelial cells. Additive effect of glucocorticoids and nedocromil sodium. Med Clin (Barc). 1997;109(1):6-11.
6. Mullol J, López E, Roca-Ferrer J, Xaubet A, Pujols L, Fernàndez-Morata JC, et al. Effects of topical anti-inflammatory drugs on eosinophil survival primed by epithelial cells. Additive effect of glucocorticoids and nedocromil sodium. Clin Exp Allergy. 1997;27(12):1432-41. http://dx.doi.org/10.1111/j.1365-2222.1997.tb02988.x PMid:9433939
7. Mullol J, Roca-Ferrer J, Xaubet A, Raserra J, Picado C. Inhibition of GM-CSF secretion by topical corticosteroids and nedocromil sodium. A comparison study using nasal polyp epithelial cells. Respir Med. 2000;94(5):428-31. http://dx.doi.org/10.1053/rmed.1999.0756 PMid:10868704
8. Xaubet A, Mullol J, Roca-Ferrer J, Pujols L, Fuentes M, Pérez M, et al. Effect of budesonide and nedocromil sodium on IL-6 and IL-8 release from human nasal mucosa and polyp epithelial cells. Respir Med. 2001;95(5):408-14. http://dx.doi.org/10.1053/rmed.2001.1061 PMid:11392584
9. Leung DY, Bloom JW. Update on glucocorticoid action and resistance. J Allergy Clin Immunol. 2003;111(1):3-22. http://dx.doi.org/10.1067/mai.2003.97
10. Pujols L, Mullol J, Roca-Ferrer J, Torrego A, Xaubet A, Cidlowski JA, et al. Expression of glucocorticoid receptor alpha- and beta-isoforms in human cells and tissues. Am J Physiol Cell Physiol. 2002;283(4):1324-31.
11. Pujols L, Mullol J, Benítez P, Torrego A, Xaubet A, de Haro J, et al. Expression of the glucocorticoid receptor alpha and beta isoforms in human nasal mucosa and polyp epithelial cells. Respir Med. 2003;97(1):90-6. http://dx.doi.org/10.1053/rmed.2002.1406 PMid:12556018
12. Hamilos DL, Leung DY, Muro S, Kahn AM, Hamilos SS, Thawley SE, et al. GRbeta expression in nasal polyp inflammatory cells and its relationship to the anti-inflammatory effects of intranasal fluticasone. J Allergy Clin Immunol. 2001;108(1):59-68. http://dx.doi.org/10.1067/mai.2001.116428 PMid:11447383
13. Knutsson PU, Brönnegård M, Marcus C, Stierna P. Regulation of glucocorticoid receptor mRNA in nasal mucosa by local administration of fluticasone and budesonide. J Allergy Clin Immunol. 1996;97(2):655-61. http://dx.doi.org/10.1016/S0091-6749(96)70311-1
14. Pujols L, Mullol J, Pérez M, Roca-Ferrer J, Juan M, Xaubet A, et al. Expression of the human glucocorticoid receptor alpha and beta isoforms in human respiratory epithelial cells and their regulation by dexamethasone. Am J Respir Cell Mol Biol. 2001;24(1):49-57. http://dx.doi.org/10.1165/ajrcmb.24.1.4024 PMid:11152650
15. Valera FC, Queiroz R, Scrideli C, Tone LG, Anselmo-Lima WT. Evaluating budesonide efficacy in nasal polyposis and predicting the resistance to treatment. Clin Exp Allergy. 2009;39(1):81-8. http://dx.doi.org/10.1111/j.1365-2222.2008.03144.x PMid:19068102
16. Bhattacharyya N, Vyas DK, Fechner FP, Gliklich RE, Metson R. Tissue eosinophilia in chronic sinusitis: quantification techniques. Arch Otolaryngol Head Neck Surg. 2001;127(9):1102-5. PMid:11556861
17. Salter M, Biggadike K, Matthews JL, West MR, Haase MV, Farrow SN, et al. Pharmacological properties of the enhanced-affinity glucocorticoid fluticasone furoate in vitro and in an in vivo model of respiratory inflammatory disease. Am J Physiol Lung Cell Mol Physiol. 2007;293(3):L660-7. http://dx.doi.org/10.1152/ajplung.00108.2007 PMid:17575011
18. Dhillon S, Wagstaff AJ. Ciclesonide nasal spray: in allergic rhinitis. Drugs. 2008;68(6):875-83. http://dx.doi.org/10.2165/00003495200868060-00009
19. Petersen H, Kullberg A, Edsbäcker S, Greiff L. Nasal retention of budesonide and fluticasone in man: formation of airway mucosal budesonide-esters in vivo. Br J Clin Pharmacol. 2001;51(2):159-63. PMid:11259988 PMCid:2014447
20. Derendorf H, Meltzer EO. Molecular and clinical pharmacology of intranasal corticosteroids: clinical and therapeutic implications. Allergy. 2008;63(10):1292-300. http://dx.doi.org/10.1111/j.13989995.2008.01750.x PMid:18782107
21. Belvisi MG, Bundschuh DS, Stoeck M, Wicks S, Underwood S, Battram CH, et al. Preclinical profile of ciclesonide, a novel corticosteroid for the treatment of asthma. J Pharmacol Exp Ther. 2005;314(2):568-74. http://dx.doi.org/10.1124/jpet.105.085217 PMid:15878996
22. van Cauwenberge P, Bachert C, Passalacqua G, Bousquet J, Canonica GW, Durham SR, et al. Consensus statement on the treatment of allergic rhinitis. European Academy of Allergology and Clinical Immunology. Allergy. 2000;55(2):116-34. http://dx.doi.org/10.1034/j.1398-9995.2000.00526.x PMid:10726726
23. Rohatagi S, Hochhaus G, Mollmann H, Barth J, Galia E, Erdmann M, et al. Pharmacokinetic and pharmacodynamic evaluation of triamcinolone acetonide after intravenous, oral, and inhaled administration. J Clin Pharmacol. 1995;35(12):1187-93. http://dx.doi.org/10.1002/j.1552-4604.1995.tb04045.x PMid:8750370
24. Johnson M. Development of fluticasone propionate and comparison with other inhaled corticosteroids. J Allergy Clin Immunol. 1998;101(4 Pt 2):S434-9. http://dx.doi.org/10.1016/S0091-6749(98)70155-1
25. Nave R, Bethke TD, van Marle SP, Zech K. Pharmacokinetics of [14C]ciclesonide after oral and intravenous administration to healthy subjects. Clin Pharmacokinet. 2004;43(7):479-86. http://dx.doi.org/10.2165/00003088-200443070-00004 PMid:15139796
26. Daley-Yates PT, Richards HR. Pharmacokinetic (PK) and pharmacodynamic (PD) relationships for intranasal corticosteroids (INCS). J Allergy Clin Immunol. 2001;107(Suppl 2):S362-S374.
27. Kaiser HB, Naclerio RM, Given J, Toler TN, Ellsworth A, Philpot EE. Fluticasone furoate nasal spray: a single treatment option for the symptoms of seasonal allergic rhinitis. J Allergy Clin Immunol. 2007;119(6):1430-7. http://dx.doi.org/10.1016/j.jaci.2007.02.022 PMid:17418384
28. Martin BG, Ratner PH, Hampel FC, Andrews CP, Toler T, Wu W, et al. Optimal dose selection of fluticasone furoate nasal spray for the treatment of seasonal allergic rhinitis in adults and adolescents. Allergy Asthma Proc. 2007;28(2):216-25. http://dx.doi.org/10.2500/aap.2007.28.2983 PMid:17479608
29. Melvin TA, Patel AA. Pharmacotherapy for allergic rhinitis. Otolaryngol Clin North Am. 2011;44(3):727-39. http://dx.doi.org/10.1016/j.otc.2011.03.010 PMid:21621057
30. Lildholdt T, Rundcrantz H, Lindqvist N. Efficacy of topical corticosteroid powder for nasal polyps: a double-blind, placebo-controlled study of budesonide. Clin Otolaryngol Allied Sci. 1995;20(1):26-30. http://dx.doi.org/10.1111/j.1365-2273.1995.tb00007.x PMid:7788929
31. Qvarnberg Y, Kantola O, Salo J, Toivanen M, Valtonen H, Vuori E. Influence of topical steroid treatment on maxillary sinusitis. Rhinology. 1992;30(2):103-12. PMid:1411095
32. Barlan IB, Erkan E, Bakir M, Berrak S, Basaran MM. Intranasal budesonide spray as an adjunct to oral antibiotic therapy for acute sinusitis in children. Ann Allergy Asthma Immunol. 1997;78(6):598-601. http://dx.doi.org/10.1016/S1081-1206(10)63223-1
33. Lavigne F, Cameron L, Renzi PM, Planet JF, Christodoulopoulos P, Lamkioued B, et al. Intrasinus administration of topical budesonide to allergic patients with chronic rhinosinusitis following surgery. Laryngoscope. 2002;112(5):858-64. http://dx.doi.org/10.1097/00005537200205000-00015 PMid:12150618
34. Lund VJ, Black JH, Szabó LZ, Schrewelius C, Akerlund A. Efficacy and tolerability of budesonide aqueous nasal spray in chronic rhinosinusitis patients. Rhinology. 2004;42(2):57-62. PMid:15224630
35. Holopainen E, Grahne B, Malmberg H, Mäkinien J, Lindqvist N. Budesonide in the treatment of nasal polyposis. Eur J Respir Dis Suppl. 1982;122:221-8. PMid:6958488
36. Tos M, Svendstrup F, Arndal H, Orntoft S, Jakobsen J, Borum P, et al. Efficacy of an aqueous and a powder formulation of nasal budesonide compared in patients with nasal polyps. Am J Rhinol. 1998;12(3):183-9. http://dx.doi.org/10.2500/105065898781390217 PMid:9653476
37. Vendelo Johansen L, Illum P, Kristensen S, Winther L, Vang Petersen S, Synnerstad B. The effect of budesonide (Rhinocort) in the treatment of small and medium-sized nasal polyps. Clin Otolaryngol Allied Sci. 1993;18(6):524-7. http://dx.doi.org/10.1111/j.1365-2273.1993.tb00628.x PMid:8877234
38. Hartwig S, Lindén M, Laurent C, Vargö AK, Lindqvist N. Budesonide nasal spray as prophylactic treatment after polypectomy (a double blind clinical trial). J Laryngol Otol. 1988;102(2):148-51. http://dx.doi.org/10.1017/S0022215100104372 PMid:3279143
39. Meltzer EO, Charous BL, Busse WW, Zinreich SJ, Lorber RR, Danzig MR. Added relief in the treatment of acute recurrent sinusitis with adjunctive mometasone furoate nasal spray. The Nasonex Sinusitis Group. J Allergy Clin Immunol. 2000;106(4):630-7. http://dx.doi.org/10.1067/mai.2000.109056 PMid:11031332
40. Nayak AS, Settipane GA, Pedinoff A, Charous BL, Meltzer EO, Busse WW, et al.; Nasonex Sinusitis Group. Effective dose range of mometasone furoate nasal spray in the treatment of acute rhinosinusitis. Ann Allergy Asthma Immunol. 2002;89(3):271-8. http://dx.doi.org/10.1016/S1081-1206(10)61954-0
41. Dolor RJ, Witsell DL, Hellkamp AS, Williams JW Jr, Califf RM, Simel DL.; Ceftin and Flonase for Sinusitis (CAFFS) Investigators. Comparison of cefuroxime with or without intranasal fluticasone for the treatment of rhinosinusitis. The CAFFS Trial: a randomized controlled trial. JAMA. 2001;286(24):3097-105. http://dx.doi.org/10.1001/jama.286.24.3097 PMid:11754675
42. Parikh A, Scadding GK, Darby Y, Baker RC. Topical corticosteroids in chronic rhinosinusitis: a randomized, double-blind, placebo-controlled trial using fluticasone propionate aqueous nasal spray. Rhinology. 2001;39(2):75-9. PMid:11486442
43. Keith P, Nieminen J, Hollingworth K, Dolovich J. Efficacy and tolerability of fluticasone propionate nasal drops 400 microgram once daily compared with placebo for the treatment of bilateral polyposis in adults. Clin Exp Allergy. 2000;30(10):1460-8. http://dx.doi.org/10.1046/j.1365-2222.2000.00932.x PMid:10998024
44. Penttilä M, Poulsen P, Hollingworth K, Holmström M. Dose-related efficacy and tolerability of fluticasone propionate nasal drops 400 microg once daily and twice daily in the treatment of bilateral nasal polyposis: a placebo-controlled randomized study in adult patients. Clin Exp Allergy. 2000;30(1):94-102. http://dx.doi.org/10.1046/j.13652222.2000.00695.x PMid:10606936
45. Lund VJ, Flood J, Sykes AP, Richards DH. Effect of fluticasone in severe polyposis. Arch Otolaryngol Head Neck Surg. 1998;124(5):513-8. PMid:9604976
46. Dijkstra MD, Ebbens FA, Poublon RM, Fokkens WJ. Fluticasone propionate aqueous nasal spray does not influence the recurrence rate of chronic rhinosinusitis and nasal polyps 1 year after functional endoscopic sinus surgery. Clin Exp Allergy. 2004;34(9):1395-400. http://dx.doi.org/10.1111/j.1365-2222.2004.02044.x PMid:15347372
47. Puhakka T, Mäkelä MJ, Malmström K, Uhari M, Savolainen J, Terho EO, et al. The common cold: effects of intranasal fluticasone propionate treatment. J Allergy Clin Immunol. 1998;101(6 Pt 1):726-31. http://dx.doi.org/10.1016/S0091-6749(98)70301-X
48. Yamada K, Elliott WM, Brattsand R, Valeur A, Hogg JC, Hayashi S. Molecular mechanisms of decreased steroid responsiveness induced by latent adenoviral infection in allergic lung inflammation. J Allergy Clin Immunol. 2002;109(1):35-42. http://dx.doi.org/10.1067/mai.2002.120525 PMid:11799363
49. Mygind N, Pedersen CB, Prytz S, Sørensen H. Treatment of nasal polyps with intranasal beclomethasone dipropionate aerosol. Clin Allergy. 1975;5(2):159-64. http://dx.doi.org/10.1111/j.1365-2222.1975.tb01848.x PMid:1095246
50. Deuschl H, Drettner B. Nasal polyps treated by beclomethasone nasal aerosol. Rhinology. 1977;15(1):17-23. PMid:335482
51. Virolainen E, Puhakka H. The effect of intranasal beclomethasone dipropionate on the recurrence of nasal polyps after ethmoidectomy. Rhinology. 1980;18(1):9-18. PMid:6988929
52. Karlsson G, Rundcrantz H. A randomized trial of intranasal beclomethasone dipropionate after polypectomy. Rhinology. 1982;20(3):144-8. PMid:6753091
53. Meltzer EO, Bachert C, Staudinger H. Treating acute rhinosinusitis: comparing efficacy and safety of mometasone furoate nasal spray, amoxicillin, and placebo. J Allergy Clin Immunol. 2005;116(6):1289-95. http://dx.doi.org/10.1016/j.jaci.2005.08.044 PMid:16337461
54. Berger W, Hampel F Jr, Bernstein J, Shah S, Sacks H, Meltzer EO. Impact of azelastine nasal spray on symptoms and quality of life compared with cetirizine oral tablets in patients with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2006;97(3):375-81. http://dx.doi.org/10.1016/S1081-1206(10)60804-6
55. Corren J, Storms W, Bernstein J, Berger W, Nayak A, Sacks H.; Azelastine Cetirizine Trial No. 1 (ACT 1) Study Group. Effectiveness of azelastine nasal spray compared with oral cetirizine in patients with seasonal allergic rhinitis. Clin Ther. 2005;27(5):543-53. http://dx.doi.org/10.1016/j.clinthera.2005.04.012 PMid:15978303
56. Horak F, Zieglmayer UP, Zieglmayer R, Kavina A, Marschall K, Munzel U, et al. Azelastine nasal spray and desloratadine tablets in pollen-induced seasonal allergic rhinitis: a pharmacodynamic study of onset of action and efficacy. Curr Med Res Opin. 2006;22(1):151-7. http://dx.doi.org/10.1185/030079906X80305 PMid:16393441
57. LaForce CF, Corren J, Wheeler WJ.; Berger WE; Rhinitis Study Group. Efficacy of azelastine nasal spray in seasonal allergic rhinitis patients who remain symptomatic after treatment with fexofenadine. Ann Allergy Asthma Immunol. 2004;93(2):154-9. http://dx.doi.org/10.1016/S1081-1206(10)61468-8
58. Ratner PH, Hampel F, Van Bavel J, Amar NJ, Daftary P, Wheeler W, et al. Combination therapy with azelastine hydrochloride nasal spray and fluticasone propionate nasal spray in the treatment of patients with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2008;100(1):74-81. http://dx.doi.org/10.1016/S1081-1206(10)60408-5
59. Lee C, Corren J. Review of azelastine nasal spray in the treatment of allergic and non-allergic rhinitis. Expert Opin Pharmacother. 2007;8(5):701-9. http://dx.doi.org/10.1517/14656566.8.5.701 PMid:17376024
60. Patel P, Wilson D, D'Andrea C, Sacks H. Onset of action of azelastine nasal spray compared to mometasone nasal spray and placebo in patients with seasonal allergic rhinitis (SAR). J Allergy Clin Immunol. 2007;119(Suppl 1):144. http://dx.doi.org/10.1016/j.jaci.2006.11.501
61. Newson-Smith G, Powell M, Baehre M, Garnham SP, MacMahon MT. A placebo controlled study comparing the efficacy of intranasal azelastine and beclomethasone in the treatment of seasonal allergic rhinitis. Eur Arch Otorhinolaryngol. 1997;254(5):236-41. http://dx.doi.org/10.1007/BF00874095 PMid:9195148
62. Yáñez A, Rodrigo GJ. Intranasal corticosteroids versus topical H1 receptor antagonists for the treatment of allergic rhinitis: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2002;89(5):479-84. http://dx.doi.org/10.1016/S1081-1206(10)62085-6
63. Pearlman DS, Grossman J, Meltzer EO. Histamine skin test reactivity following single and multiple doses of azelastine nasal spray in patients with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2003;91(3):258-62. http://dx.doi.org/10.1016/S1081-1206(10)63527-2
64. Altounyan RE. Review of clinical activity and mode of action of sodium cromoglycate. Clin Allergy 1980;10(suppl):481-9.http://dx.doi.org/10.1111/j.1365-2222.1980.tb02162.x PMid:6821510
65. Cox JS, Beach JE, Blair AM, Clarke AJ, King J, Lee TB, et al. Disodium cromoglycate (Intal). Adv Drug Res. 1970;5:115-96. PMid:4099491
66. Pelikan Z, Snoek WJ, Booij-Noord H, Orie NG, de Vries K. Protective effect of disodium cromoglycate on the allergen provocation of the nasal mucosa. Ann Allergy. 1970;28(11):548-53. PMid:5000143
67. Storms W, Kaliner MA. Cromolyn sodium: fitting an old friend into current asthma treatment. J Asthma. 2005;42(2):79-89. http://dx.doi.org/10.1081/JAS-52017
68. Ratner PH, Meltzer EO, Byas L, Block EM. Randomized, double-blind, placebo-controlled evaluation of cromolyn sodium in the treatment of allergic rhinitis in young children. J Appl Res. 2001;1:100-9.
69. Meltzer EO; NasalCrom Study Group. Efficacy and patient satisfaction with cromolyn sodium nasal solution in the treatment of seasonal allergic rhinitis: a placebo-controlled study. Clin Ther. 2002;24(6):942-52. http://dx.doi.org/10.1016/S0149-2918(02)80009-1
70. Schatz M, Zeiger RS, Harden K, Hoffman CC, Chilingar L, Petitti D. The safety of asthma and allergy medications during pregnancy. J Allergy Clin Immunol. 1997;100(3):301-6. http://dx.doi.org/10.1016/ S0091-6749(97)70241-0
71. Kushnir NM. The role of decongestants, cromolyn, guafenesin, saline washes, capsaicin, leukotriene antagonists, and other treatments on rhinitis. Immunol Allergy Clin North Am. 2011;31(3):601-17. http://dx.doi.org/10.1016/j.iac.2011.05.008 PMid:21737044
72. Ratner PH, Ehrlich PM, Fineman SM, Meltzer EO, Skoner DP. Use of intranasal cromolyn sodium for allergic rhinitis. Mayo Clin Proc. 2002;77(4):350-4. http://dx.doi.org/10.4065/77.4.350 PMid:11936930
73. Neffen H, Mello JF Jr, Sole D, Naspitz CK, Dodero AE, Garza HL, et al. Nasal allergies in the Latin American population: results from the Allergies in Latin America survey. Allergy Asthma Proc. 2010;31 Suppl 1:S9-27. http://dx.doi.org/10.2500/aap.2010.31.3347 PMid:20557683
74. Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al.; Global Allergy and Asthma European Network; Grading of Recommendations Assessment, Development and Evaluation Working Group. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126(3):466-76. http://dx.doi.org/10.1016/j.jaci.2010.06.047 PMid:20816182
75. Josling P, Steadman S. Use of cellulose powder for the treatment of seasonal allergic rhinitis. Adv Ther. 2003;20(4):213-9. http://dx.doi.org/10.1007/BF02850092
76. Emberlin JC, Lewis RA. A double blind, placebo controlled trial of inert cellulose powder for the relief of symptoms of hay fever in adults. Curr Med Res Opin. 2006;22(2):275-85. http://dx.doi.org/10.1185/030079906X80440 PMid:16466599
77. Emberlin JC, Lewis RA. A double blind, placebo-controlled cross over trial of cellulose powder by nasal provocation with Der p1 and Der f1. Curr Med Res Opin. 2007;23(10):2423-31. http://dx.doi.org/10.1185/030079907X231144 PMid:17767803
78. Harvey R, Hannan SA, Badia L, Scadding G. Nasal saline irrigations for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev. 2007;18;(3):CD006394.
79. Marple B, Roland P, Benninger M. Safety review of benzalkonium chloride used as a preservative in intranasal solutions: an overview of conflicting data and opinions. Otolaryngol Head Neck Surg. 2004;130(1):131-41. http://dx.doi.org/10.1016/j.otohns.2003.07.005 PMid:14726922
80. Bernstein IL. Is the use of benzalkonium chloride as a preservative for nasal formulations a safety concern? A cautionary note based on compromised mucociliary transport. J Allergy Clin Immunol. 2000;105(1 Pt 1):39-44. http://dx.doi.org/10.1016/S00916749(00)90175-1
81. Meltzer EO. Formulation considerations of intranasal corticosteroids for the treatment of allergic rhinitis. Ann Allergy Asthma Immunol. 2007;98(1):12-21. http://dx.doi.org/10.1016/S1081-1206(10)60854-X
82. Heatley DG, McConnell KE, Kille TL, Leverson GE. Nasal irrigation for the alleviation of sinonasal symptoms. Otolaryngol Head Neck Surg. 2001;125(1):44-8. http://dx.doi.org/10.1067/mhn.2001.115909 PMid:11458213
83. Brown CL, Graham SM. Nasal irrigations: good or bad? Curr Opin Otolaryngol Head Neck Surg. 2004;12(1):9-13. http://dx.doi.org/10.1097/00020840-200402000-00004
84. Georgitis JW. Nasal hyperthermia and simple irrigation for perennial rhinitis. Changes in inflammatory mediators. Chest. 1994,106(5):1487-92. http://dx.doi.org/10.1378/chest.106.5.1487 PMid:7956408
85. Kim D, Baraniuk JN. Neural aspects of allergic rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2007;15(4):268-73. http://dx.doi.org/10.1097/MOO.0b013e328259c372 PMid:17620902
86. Talbot AR, Herr TM, Parsons DS. Mucociliary clearance and buffered hypertonic saline solution. Laryngoscope. 1997;107(4):500-3. http://dx.doi.org/10.1097/00005537-199704000-00013 PMid:9111380
87. Min YG, Lee KS, Yun JB, Rhee CS, Rhyoo C, Koh YY, et al. Hypertonic saline decreases ciliary movement in human nasal epithelium in vitro. Otolaryngol Head Neck Surg. 2001;124(3):313-6. http://dx.doi.org/10.1067/mhn.2001.113145 PMid:11240998
88. Boek WM, Keleş N, Graamans K, Huizing EH. Physiologic and hypertonic saline solutions impair ciliary activity in vitro. Laryngoscope. 1999;109(3):396-9. http://dx.doi.org/10.1097/00005537-19990300000010 PMid:10089964
89. Kim CH, Hyun Song M, Eun Ahn Y, Lee JG, Yoon JH. Effect of hypo-, iso- and hypertonic saline irrigation on secretory mucins and morphology of cultured human nasal epithelial cells. Acta Otolaryngol. 2005;125(12):1296-300. http://dx.doi.org/10.1080/00016480510012381 PMid:16303677
90. Homer JJ, England RJ, Wilde AD, Harwood GR, Stafford ND. The effect of pH of douching solutions on mucociliary clearance. Clin Otolaryngol Allied Sci. 1999;24(4):312-5. http://dx.doi.org/10.1046/ j.1365-2273.1999.00265.x PMid:10472466
91. Yu S, Zhao Y, Wu F, Zhang X, Lü W, Zhang H, et al. Nasal insulin delivery in the chitosan solution: in vitro and in vivo studies. Int J Pharm. 2004;281(1-2):11-23. http://dx.doi.org/10.1016/j.ijpharm.2004.05.007 PMid:15288339
92. Wingertzahn MA, Sato H, Nave R, Nonaka T, Mochizuki T, Takahama S, et al. Comparison of nasal tissue concentrations in rabbits following administration of hypotonic and isotonic ciclesonide suspensions. J Allergy Clin Immunol. 2005;115(Suppl 2):S126. http://dx.doi.org/10.1016/j.jaci.2004.12.516
93. Olson DE, Rasgon BM, Hilsinger RL Jr. Radiographic comparison of three methods for nasal saline irrigation. Laryngoscope. 2002;112(8 Pt 1):1394-8. http://dx.doi.org/10.1097/00005537-200208000-00013 PMid:12172251
94. Benninger MS, Hadley JA, Osguthorpe JD, Marple BF, Leopold DA, Derebery MJ, et al. Techniques of intranasal steroid use. Otolaryngol Head Neck Surg. 2004;130(1):5-24. http://dx.doi.org/10.1016/j.otohns.2003.10.007 PMid:14726906
95. Mello Junior JF. Higiene nasal e soluções salinas. In: Sole D, Prado E, Mello Junior JF, editores. Rinite Alérgica: conhecendo melhor. 1a edição. São Paulo: Conexão editorial; 2010. p.218-25.
96. Xiong GX, Zhan JM, Jiang HY, Li JF, Rong LW, Xu G. Computational fluid dynamics simulation of air flow in the normal nasal cavity and paranasal sinuses. Am J Rhinol. 2008;22(5):477-82. http://dx.doi.org/10.2500/ajr.2008.22.3211 PMid:18954506
1. Senior Associate Professor - Medical School of the University of São Paulo (FMUSP).
2. Professor/Collaborator at the Otorhinolaryngology Program - University of São Paulo.
3. PhD in Otorhinolaryngology - Medical School of the University of São Paulo.
4. Associate professor - Medical School of Ribeirão Preto - University of São Paulo.
5. MSc in Otorhinolaryngology and PhD in Medicine - Federal University of São Paulo.
6. Professor/Coordinator of the Otorhinolaryngology Medical Residency Program - Federal University of Rio Grande do Norte.
7. PhD in Otorhinolaryngology - Federal University of São Paulo. Head of the Otorhinolaryngology Department - Santa Casa da Misericórdia - Rio de Janeiro.
8. PhD in Sciences - FMUSP, Collaborator Physician - Pediatric Otorhinolaryngology Program - Federal University of São Paulo.
9. PhD in Sciences - Medical School - University of São Paulo (FMUSP).
10. Associate Professor of Otorhinolaryngology - Medical School of the Lutheran University in Brazil. Associate Scientific Staff - Department of Otolaryngology Mount Sinai Hospital Toronto Ontario Canada.
11. Senior Associate Professor - State University of Rio de Janeiro.
12. MSc in Otorhinolaryngology - Federal University of Rio de Janeiro. Professor/Collaborator - Otorhinolaryngology Program - School of Medical Sciences - State University of Rio de Janeiro.
13. Head of the Otorhinolaryngology Service - Policlínica de Botafogo - Rio de Janeiro.
14. MD. Otorhinolaryngologist.
15. Associate Professor - Medical School of the Federal University of Minas Gerais. Senior Associate Professor - University of São Paulo/Ribeirão Preto.
Send correspondence to:
João Ferreira de Mello Jr
Rua Dr. Eneas de Carvalho Aguiar, nº 255, 6º andar
São Paulo - SP. Brazil. CEP: 05403-000
Paper submitted to the BJORL-SGP (Publishing Management System - Brazilian Journal of Otorhinolaryngology) on November 30, 2011.
Accepted on October 7, 2012. cod. 8928.