Year: 2002 Vol. 68 Ed. 4 - (6º)
Artigo Original
Pages: 488 to 494
Audiometric evaluation of noise and cadmium occupationally exposed workers
Author(s):
Mauricio T. de Abreu 1,
Fábio A Suzuki 2
Keywords: noise, cadmium, noise induced hearing loss, audiometry, occupational exposure
Abstract:
Introduction: Noise is the most common harmful agent present in industrial environments, leading to hearing loss. Besides noise, chemical agents also present in industries, such as solvents and metals lead to hearing loss. Aim: Evaluation of two groups of workers exposed to industrial noise, one of these groups was exposed to cadmium and the other was not, and verify if the concomitant exposition to cadmium and noise is more harmful than the exposition only to noise. Material and Method: It was realized an audiometric research with 36 employees in a metallurgical industry, who were exposed to noise and cadmium fumes at their work environment, from 1994 to 1998. The obtained results in this group were compared to a control group composed by 36 employees, from the same areas of the factory, therefore exposed only to noise. Results: The analysis of the obtained results showed an auditory damage, mainly at the frequencies of 4000 and 6000 Hz, stronger at the group exposed to noise and cadmium. Conclusion: The results showed a probable ototoxic action of the metal when it is associated with noise exposition.
![]()
INTRODUCTION
The progress in various areas of human knowledge has created a noisier society. The so-called sound pollution is the most common type of pollution in the modern world.
Noise has been considered the main causing factor of auditory abnormalities in people who work in the industry, and there are reports of affections in different line of business. Clinical characteristics of noise-induced hearing loss are insidious onset and irreversibility1.
Noise is considered the most frequent health damaging agent in the workplace4, 27.
It is admitted that even though it has become an increasingly important problem, the acknowledgement of noise-induced hearing loss is not universal5.
Noise is still characterized as the most prevalent factor in the origin of occupational diseases and it is also the most common health damaging agent in the workplace3, 22.
We are all subject to exposure to potentially damaging sounds in our daily lives and it is believed that noise-induced hearing loss is the main isolated cause of hearing loss that is preventable28.
The main clinical characteristic of long lasting hearing loss induced by noise exposure is its insidious onset. In addition, it is normally symmetrical and the loss is concentrated in frequencies between 3 and 6 kHz1.
Noise-induced hearing loss is classified by the National Committee on Noise and Auditory Conservation10 as the gradual reduction of auditory acuity, resulting from continuous exposure to high levels of noise.
It is believed that noise can cause extra-auditory abnormalities, such as vestibular abnormalities, high arterial blood pressure, different neurological disorders, gestation affections, problems in the excretion of cortisol, among others, even though there are no definite evidence of these effects.
Many chemicals can cause morphological and functional damage to the inner ear. The presence of noise associated to these agents can be an aggravating factor of the impairment of the ear.
Solvents and metals are considered risk factors when associated to noise. The effects of these substances in general are not very well defined and the diagnosis is based on confirmation of exposure and presence of signs and symptoms.
The presence of noise combined to some of these ototoxic agents can be potentially more harmful than when isolated6.
Many studies concluded that exposure to solvents is a significant relative risk of hearing loss when compared to people not exposure to chemicals18.
The ototoxic mechanism of solvents has not been fully understood yet and requires further study. This association is rarely studied and despite the great number of workers exposure to ototoxic chemicals, only a small percentage of those who are exposed to noise will be audiologically tested regularly.
Among solvents, toluene was studied in a Brazilian company of printing and paints, dividing workers into four groups: not exposed, exposed to noise, exposed to toluene and exposed to both noise and toluene. The risk of having a hearing loss decreased in the following order: the group exposed to both toluene and noise, only to toluene, only to noise and, finally, those not exposed23.
In literature review studies about noise and organic solvent occupational exposure and the effects on the hearing system, three confirmed neurototoxic agents were evidenced: toluene, trichloro ethylene and carbon disulfide. Two other agents were highlighted as probably neurotoxic: styrene and xylene. However, the authors emphasized the need for further studies24.
Other authors reviewed the great number of recently conducted studies that investigated the potential effects of the combination between noise and different industrial substances. The agent most often referred are organic solvents (carbon disulfide, toluene, styrene, trichloro ethylene, and others), carbon monoxide and metals (lead, cadmium, manganese and aluminum)9.
The ototoxic effect of carbon monoxide was determined in audiometric and electronystagmographic tests performed in occupationally exposed workers. In 66.6% of the cases we found auditory abnormalities and in 79.5% of them, vestibular abnormalities21.
Other agents, such as for example antibiotics are mentioned. In studies with rats it was shown that chloramphenicol isolated did not produce cochlear damage. The combination with noise exposure caused more cochlear damage if compared to isolated noise exposure8.
In the industry, we could observe a number of risk factors. Depending on the exposure characteristics, chemicals such as solvents and metals can cause auditory abnormalities, regardless of the presence of noise24.
NOISE, EXPOSURE TO METALS AND HEARING DISORDERS
In a study with workers exposed to metallic fumes of manganese the authors found abnormalities in the audiometric tests and vestibular indexes in subjects with minimum level of metal exposure. The authors suggested that the test was a significant criterion for the early diagnosis of chronic manganese intoxication20.
Toxicology of metals comprises a great field of research in which occupational studies can be developed. Most metals are not essential for human beings, but they can produce toxic effects as a result of daily absorption of small doses for prolonged periods of time. Absorption, distribution, retention and excretion by the body vary according to the metal. Environment pollution to metallic elements and subsequent human exposure can occur in different forms at occupational levels, and industrial applications of metals have continuously increased2.
In recent years, a significant number of studies have been conducted to investigate the potential effects to the body of the combination noise and metals, especially lead and cadmium.
In a recent study with workers exposed to lead and noise there was statistically significant correlation and the results suggested that there is interaction between the two factors, but further assessment is required16.
Cadmium poisoning has gained attention owing to its importance both from the occupational and the environmental perspectives. Cadmium exposure is noticed in workers of industries that manufacture batteries and in some groups of welders and welding machine operators. Exposure to cadmium and noise simultaneously seem to be more harmful than exposure to either one isolated.
Cadmium has been found in different organs, such as pancreas, testis, thyroid, salivary glands, heart and others.
Kidney is the primary organ that reaches critical concentration of cadmium as a result of metal accumulation. Renal cortex is the region in which cadmium is more concentrated. Cadmium excretion is very slow and occurs mainly through the urinary and intestinal routes. Urinary excretion of cadmium increases with aging. Other excretion routes are described, but they are less important, such as for example the salivary route.
In workers exposed to cadmium, the main affection in renal damage is caused by lesion of the proximal tubule, affecting the reabsorption function and later, of the distal tubule and glomeruli. The first signs are related to increased urinary excretion of low molecular weight proteins. Even though there are many models trying to elucidate the toxicity mechanism of cadmium, such mechanism has not been fully understood yet. Cadmium intoxication is characterized by renal damage with proteinuria 26.
Findings in lab animals provided significant information to nephrotoxic effects. After 15 days of exposure, there are the first ultra-structural damages, all of them located in the proximal tubules. After two months, there are proximal tubule lesions with edema and cytoplasm vacuolization of microvilli. After three months, glomerular modifications become perceptive, such as Bowman capsule and basal membrane thickness. Distal tubule damage is noticed after the 20th week of exposure12.
Pulmonary abnormalities found in workers exposed to cadmium, compared to groups that were not exposed, consisted of a large number of abnormal functions and radiological abnormalities that were consistent with emphysema. Abnormalities were more marked in those exposed to the metal for longer periods of time. There is evidence that cadmium causes this disease11, 14.
Cadmium occupational exposure has been poorly studied in Brazil, although import of the metal has increased significantly. The number of exposed workers is unknown, but the growing consumption of the metal suggests that the exposure is significant12.
Respiratory route is described as the main route that introduces the metal into the body within the industrial environment13. The amount of cadmium absorbed by this route depends on many factors:
1. size, chemical form and solubility of retained particles;
2. amount of deposited metal, and
3. depuration mechanism.
About 70% of the cadmium absorbed is found in the erythrocytes, bound to hemoglobin. After absorption, cadmium is fixed to a protein, metallothionein, which is synthesized by the liver. Owing to its low molecular weight, the protein is filtered through the glomeruli and reabsorbed by proximal tubule cells in which it releases the metal12.
In men, some toxic effects resulting from prolonged exposure to cadmium are related to kidneys, lungs, bones and liver, among others14.
The effects of cadmium in the nervous system have also been discussed, knowing to affect various areas: the visual system, olfactory disorders, polyneuropathies and various neurological abnormalities.
Neuropsychological effects were attributed to cadmium exposure, such as memory disorders, cognitive deficit, psychomotor speed, among others. Cadmium interferes in various important functions of the nervous system, but the neurotoxicity mechanisms are still undefined9.
Olfaction abnormalities are described in workers chronically exposed to cadmium fumes, and the hyposmia was found in degrees that ranged from mild to severe25.
Renal damage induced by cadmium is irreversible. It was evidenced that the glomerular damage progresses even after interruption of exposure to the metal19.
Experimental laboratory studies suggested the existence of noise exposure associated to cadmium and that it would increase the toxic effect of the latter9.
It has been demonstrated that cadmium can be a potent neurotoxic agent, especially for the peripheral nervous system. Half-life of the metal in the organism is greater than 15 years and polyneuropathy can develop as years go by, even after end of exposure. Findings have pointed favorably to the hypothesis that cadmium has a role in the development of polyneuropathy 29.
In a recent study about neurological abnormalities related to cadmium, complaints compatible with peripheral neuropathy and systemic nerve dysfunctions were detected in workers exposed to the metal, followed by balance and concentration skill disorders. In the study, the authors described these abnormalities before any signs of renal abnormalities were detected30.
The knowledge available to understand the occupational aspects of cadmium exposure is still limited, and in some occasions it is necessary to infer data from epidemiological studies and animal trials. Toxicokinetics of cadmium in the body involves complex aspects and there are many questions whose answers still depend on research studies, which are normally technically difficult to conduct, but nevertheless very necessary.
OBJECTIVE
To assess two groups of subjects exposed to the same levels of industrial noise, being one group exposed to cadmium as well, and to check whether the exposure to both pollutant agents is more harmful than the exposure to noise alone.
MATERIAL AND METHOD
In the present study we used audiometry assessment (pure tone audiometry) in 36 workers from a metallurgical company in the region of Greater ABC, São Paulo, in areas exposed to noise and metallic fumes rich in cadmium. We organized a control group with 36 workers from the same sectors of the company who were exposed only to noise.
The study was conducted in a 5-year period (between 1994 and 1998).
The studied workers were aged on average 37.16 years, minimum age of 23 years and maximum age of 48 years at the beginning of the study. In the control group, the mean age was 37.02 years, minimum age of 22 years and maximum of 47 years, also at the beginning of data collection.
In the group, we studied 32 welders and 4 operators of hand torch from four different sectors of the company. In the control group, we included workers from the same sectors, but who were not exposed to welding fumes. In both groups, no workers reported renal function compromise in the medical and laboratory history.
Noise levels were measured by a specialized company, performed at different sites of the workstation, scale A and in the slow response circuit, as provided by Annex 1, Regulating Standard (NR-15) of Administrative Rule 3214/78. A sound pressure level meter brand Simpson model 884 was used, after acoustic calibration. Noise levels varied from 80 to 118dB15.
We collected samples of metallic fumes and dust with special filters, following the appropriate methodology. Samples were dosed gravimetrically and then using atomic absorption spectrophotometry. All results of evaluations were below the limits set by the legislation, according to Annex 12, NR-15 of Administrative Rule 321415.
Audiometric tests were performed with audiometer MAICO - MA 41 (standard ANSI-69), calibrated every six months by a specialized company. We also used the sound proof booth in compliance with the standards, built by a specialized company.
Tests were performed in 1994, 1995, 1996, 1997 and 1998 in different groups, according to the month the employee had joined the company, at regular intervals of 12 months.
All tests were performed by the author, with auditory rest for over 14 hours, according to the standard of the National Committee on Noise and Auditory Conservation. We used frequencies 250, 500, 1000, 2000, 3000, 4000, 6000 and 8000 Hz, on both ears. We excluded all subjects who presented conductive hearing loss.
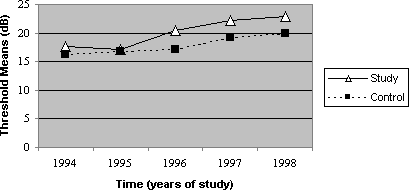
Graph 1. Mean values of thresholds according to time, 4000Hz frequency, on the right ear, for the control and study groups.
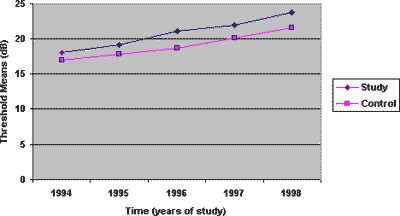
Graph 2. Mean values of thresholds according to time, 4000 Hz frequency, on the left ear, for the study and control group.
RESULTS
The results were analyzed from different statistical perspectives (arithmetic mean, standard deviation, absolute variation and percentage means, non-paired t test, paired t-test).
In the analysis of results, upon the mean of all frequencies for five years in the 36 studied subjects, we did not find statistically significant differences for frequencies and ears in both groups.
In the group analysis, by observing the data for year 1994 (before) and 1998 (after), we detected statistically significant differences for frequencies and ears in both groups.
By observing the mathematical means of frequencies and years, we noticed that the greatest loss was present in 4000Hz.
Next, we conducted variance analysis of repetitive measures with factors, all frequencies and data between the groups - the control and the study. We detected variations between frequencies, but no variations between ears and groups (Table 1).
We then employed only the frequencies that showed great variation (2000, 3000, 4000 and 6000Hz) to calculate variance analysis for repetitive measures with factors. The calculation of the analysis showed significant joint variation for frequencies, ears and groups (Table 2).
We also simultaneously performed Tuckey and SNK test (Student Newmam Keuls) and variations were located between groups in frequency 4000Hz, right ear (Tuckey) and both ears (SNK). For 6000Hz frequency, we observed significant variation on the right side in SNK test (Table 3).
Therefore, the results of the variance analysis with repetitive measures with factors (Tables 1 and 2) and the multiple comparison tests of Tuckey and SNK (Table 3) showed statistically significant variation for frequencies 4000Hz and 6000Hz, but especially the former.
Table 1. Variance analysis for repetitive measures, with factors for 2 groups,. 8 frequencies and 2 sides. F calculated and its significance (*).
Table 2. Variance analysis for repetitive measures, with factors for 2 groups, 4 frequencies (2000, 3000, 4000 and 6000Hz) and 2 sides. F calculated and its significance (*).
Table 3. P values calculated in Tuckey and SNK test for variations between control and studied groups, frequencies 2000, 3000, 4000 and 6000 Hz and both sides.
DISCUSSION
In addition to noise, other agents present in the occupational environment seem to influence the auditory system. Chemicals such as solvents and heavy metals are described as the causing agents of hearing disorders.
Studies addressing the topic are not very frequent when compared to those that address noise. Generally speaking, they are studies that compare groups exposed to the chemicals to groups of people not exposed to them. Very little is said about histology abnormalities in the inner ear and the correlation between long term observation of partial loss of hearing function associated to exposure to the agent.
The joint action of noise and chemicals and metals is nearly never discussed.
The present study compared two groups of subjects who were exposed to occupational noise and one of them was also exposed to cadmium fumes.
The absence of studies about ototoxic abnormalities induced by cadmium in association to noise conducted for longer periods of time, raised the idea of the present study, in which the same subjects were analyzed at annual intervals in five audiometry verifications, performed always with the same equipment, in the same sound-proof booth and by the same examiner. There was, hence, no interference from the adopted method.
Upon forming the groups, we included 36 workers of 4 sectors of the company who were exposed to noise and to cadmium fumes. Other 36 workers from the same sectors, not exposed to cadmium, but exposed to the same intensity of noise, were selected to the control group.
The subjects for the control group were selected based on age, so as to have age-matched groups.
Extreme age has been referred as risk factor for ototoxicity 7. This factor was not present in this study in which the mean age was 37.16 years in the study group and 37.02 years in the control group.
In the group analysis, upon observing the data from 1994 (before) and 1998 (after), there was statistically significant difference present in the study for frequencies and ears in both groups. Therefore, the interference of noise as a factor that impairs hearing can be easily demonstrated.
The National Committee on Noise and Hearing Conservation noticed that in NIHL the initial loss is concentrated in 3000, 4000 and 6000Hz, before affecting the other frequencies. It has also been emphasized the maximum loss in 3000, 4000 and 6000Hz in the first ten to fifteen years of exposure2.
In our study, the most frequent loss was in 4000Hz. which is the most frequently affected frequency in NIHL. The results of multiple variance analysis showed statistically significant variation in frequencies 4000 and 6000Hz, especially in the former. Therefore, it seems that cadmium interacts with noise and maximizes the effect of noise, aggravating the difference of hearing loss in such frequencies when compared to the group exposed only to noise.
The correlation between exposure to cadmium fumes and noise as being more harmful to the hearing system, when compared to noise alone, has not been fully determined yet, because no study has demonstrated in which site the metal acts, nor its action mechanism.
Cadmium interferes in various important functions of the nervous system, but the toxicity mechanisms remain uncertain17.
The thresholds of 4000Hz presented greater different between the noise exposed and the noise and cadmium exposed groups, as of the second audiometric assessment, a trend that remained in subsequent years (Graphs 1 and 2). Based on these data, it is supposed that the toxic action of cadmium is greater after some time of exposure, but it stabilizes and the difference between the groups is maintained in subsequent years. Thus, it is possible to believe that toxicity seems to be smaller at the beginning of the exposure, and it aggravates as time goes by, when there is metal deposit in the subject's body. Based on studies of toxic potential of cadmium, we can conclude that once the exposure is interrupted, the toxic effect can not be eliminated, and the continuity of noise exposure can result in progression of hearing loss.
By making a comparison between renal lesions caused by cadmium and other nephrotoxic agents, such as for example, aminoglycoside antibiotics, we observed a similarity between them, with higher frequency of renal damage caused by cadmium as a lesion located in the proximal tubule13. Renal damage associated to nephrotoxic aminoglycoside antibiotics normally affects the proximal tubule, which is considered the most common site of lesion7.
The first step of ototoxic effect of aminoglycoside is electrostatic interaction with hair cell membrane. The aminoglycoside is then transported into the cell. Once inside, it can interfere in various intracellular reactions, leading to progression of ototoxic effects. Similarly, would it be the same ototoxic mechanisms for cadmium? Could the abnormality be detected at the level of the brainstem? Studies in animals can help clarifying these issues.
The number of ototoxic agents and factors that act in synergy has increased. In addition, various factors interfere to determine the risk level. Such factors are not only exposure time, age, other drugs in use, individual variability. Concomitant noise exposure seems to be described as an aggravating factor for chemical-induced hearing loss.
In the present study, there was statistically significant difference in audiometric abnormalities in groups of subjects exposed to cadmium and noise when compared to the group of subjects exposed only to noise, suggesting the interaction between the two elements.
The knowledge about the final effects of noise combination with a number of industrial substances indicates the limited amount of information available, owing to lack or failure in studies with animal models and the lack of knowledge about the actions of different substances in the body and its interactions with noise9.
Half-life of cadmium in the body is very long, causing an accumulation during the lifetime. Therefore, even after metal exposure ceases, it is accumulated in the body, being slowly eliminated. Animal trials are very limited because the action of different agents is normally subject to great variations in each specific species, hindering standardization of study models7. The few studies in animals related to the effects of cadmium associated to noise are restricted to renal effects, neurotoxicity and abnormalities in the formation of bone skeleton9. The existence of audiometric evidence in the present study emphasizes the need to conduct controlled experimental studies in animals to try to identify the most probable otological alteration site.
Exposure to cadmium can be monitored through blood and urine dosing. It was noticed that there may be neurological abnormalities in subjects exposed to cadmium even in the presence of preserved renal function and low concentrations of metals in the available lab tests30.
In order to try to ensure that no subject exposed to potentially ototoxic agents and noise, or without noise exposure, develop hearing impairment, we recommend periodical hearing tests. By doing so, minor variations of hearing function will be detected.
CONCLUSION
The analysis of the results obtained from 36 workers exposed to occupational cadmium fumes and noise and a control group with 36 workers exposed only to noise led to the following conclusions:
1. Occupational noise exposure resulted in hearing impairment in the control group.
2. Noise exposure led to hearing impairment mainly in 4000 and 6000Hz frequencies.
3. It was demonstrated that the association between occupational noise and cadmium fume exposure led to more severe hearing impairment than isolated noise exposure.
References
1. Alberti, PW. Deficiência Auditiva Induzida pelo Ruído. In: Lopes Filho O & Campos CAH. Tratado de Otorrinolaringologia. São Paulo: Ed. Roca; 1994. p. 93.
2. Alessio L, Apostolli P, Della Rosa HV. Toxicology of metals. Rev Bras Toxicol 1991;4(1/2):1-5, 4-949.
3. Almeida SIC. Efeitos lesivos do ruído e implicações legais. Acta AWHO 1993;12(3):121-127.
4. Azevedo AP, Okamoto Va, Bernardi RA. Considerações sobre Ruído: Riscos, Patologia e Prevenção. In: Costa DF, Carmo JC, Settimi MM, Santos UP. Programa de Saúde dos trabalhadores: A Experiência da Zona Norte: Uma Alternativa em Saúde Pública. São Paulo: Ed. Hucitec; 1989. cap. 5, p. 83-155.
5. Bahadori Rs, Bohne BA. Adverse Effects of Noise on Hearing. Am Fam Physician 1993;47(5):1219-1229.
6. BHATTACHARYYA, TK; DAYAL, VS. Ototoxicity and noise-drug interaction. J Otolaryngol 1984;13(6):361-366.
7. Black FO & Pesznecker SC. Vestibular ototoxicity. Otolaryngol Clin North Am 1993;26(5):713-736.
8. Brown RD, Penny JE, Henley CM, Hodges KB Kupetz SA, Glenn DW, Jobe JC. Ototoxic drugs and noise. In: Ciba Found Symp 1981;85:151-171.
9. Cary R, Clarke S, Delic J. Effects of combined exposure to noise and toxic substances - Critical review of the literature. Ann Occup Hyg 1997;41(4):455-465.
10. Comitê Nacional De Ruído E Conservação Auditiva. Perda Auditiva Induzida pelo Ruído Relacionada ao Trabalho. Acta AWHO 1994;13(3):126-127.
11. Davison AG, Fayers PM, Taylor AJ, Venables KM, Darbyshire J, Pickering CA, Chettle DR, Franklin D, Guthrie CJ, Scott MC. Cadmium fume inhalation and emphysema. Lancet 1988;26(1):663-667.
12. Della Rosa H, Gomes JR. Monitorização Biológica de Trabalhadores Expostos ao Cádmio. Rev bras saúde ocup 1988;16(61):49-52.
13. Della Rosa H, Gomes JR. Cádmio - Toxicocinética. Rev bras saúde ocup 1988;16(61):39-42.
14. Della Rosa H, Gomes JR. Cádmio: Efeitos Patológicos. Rev bras saúde ocup 1988;16(61):43-48.
15. Ecolabor Comercial Consultoria E Análises Ltda. Avaliação Ambiental - Confab Tubos S/A. São Paulo; 1995. 48 pág.
16. Forst LS, Freels S, Persky V. Occupational lead exposure and hearing loss. J Occup Environ Med 1997;39(7):658-660.
17. Hart RP, Rose CS, Hamer RM. Neuropsychological effects of occupational exposure to cadmium. J Clin Exp Neuropsychol 1989;11(6):933-943.
18. Jacobsen P, Hein Ho, Suadicani P, Parving A, Gyntelberg F. Mixed solvent exposure and hearing impairment: an epidemiological study of 3284 men. The Copenhagen male study. Occup Med (Lond) 1993;43(4):180-184.
19. Jarup L, Persson B, Elinder CG. Decreased glomerular filtration rate in solderers exposed to cadmium. Occup Environ Med 1995;52(12):818-822.
20. Khalkova Z, Kostadinova G. Auditory-vestibular changes in workers in ferrous metallurgy manufacture. Probl Khig 1986;(11):134-138.
21. Kowalska S. State of the hearing and equilibrium organs in workers exposed to carbon monoxide. Med Pr 1981;32(2):145-151.
22. Kwitko A, Koch E. Audiometria industrial de "screening": conceitos e bases de um programa de gerenciamento de dados. Acta AWHO 1994;13(3):90-98.
23. Morata TC, Dunn DE, Kretschmer LW, Lemasters GK, Keith RW. Effects of occupational exposure to organic solvents and noise and hearing. Scand J Work Environ Health 1993;19(4):245-254.
24. Morata TC, Dunn DE, Sieber WK. Occupational exposure to noise and ototoxic organic solvents. Arch Environ Health 1994;49(5):359-365.
25. Rose CS, Heywood PG, Costanzo RM. Olfactory impairment after chronic occupational cadmium exposure. J Occup Med 1992;34(6):600-605.
26. Scott R, Mills EA, Fell GS,, Husain FER, Yates AJ, Paterson PJ, Mckirdy A, Otto-Way JM, Fitzgerald-Finch OP, Lamont A, Roxburgh S. Clinical and biochemical abnormalities in coppers smiths exposed to cadmium. Lancet London 1976;(2):396-398.
27. Seligman J, Ibañez RN. Considerações a respeito da perda auditiva induzida pelo ruído. Acta AWHO 1993;12(2):75-79.
28. Stangler S, Souza EOH, Gomes NHG, Seligman J, Moussalle SK. Perda auditiva induzida por ruído. Acta méd 1995 (Porto Alegre)(1):253-261.
29. Viaene MK, Roels HA, Leenders J, De Groof M, Swerts LJ, Lison D, Masschelein R. Cadmium: a possible etiological factor in peripheral polyneuropathy. Neurotoxicology 1999;20(1):7-16.
30. Viaene MK, Masschelein R, Leenders J, Degroof M, Swerts LJ, Roels HA. Neurobehavioural effects of occupational exposure to cadmium: a cross sectional epidemiological study. Occup Environ Med 2000;57(1):19-27.
[1] Instructor, Medical School, ABC.
[2] Ph.D. in Otorhinolaryngology, UNIFESP, Deputy-Coordinator of Post-Graduation Program in Otorhinolaryngology, Post-Graduation Committee, Hospital do Servidor Público Estadual - IAMSPE - SP
Study based on the Master Degree Dissertation Thesis submitted to the Post-Graduation Committee, Hospital do Servidor Público Estadual - IAMSPE - SP, and approved on August 24, 2000.
Address correspondence to: Praça Cardeal Arcoverde, 19 - São Caetano do Sul - São Paulo -09510-030. Tel: (55 11) 4227.5862; Fax: (55 11) 4229.1252 - E-mail: mtabreu@uol.com.br