Year: 2012 Vol. 78 Ed. 6 - (10º)
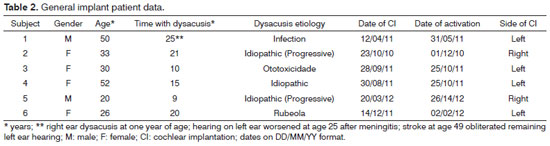
Artigo Original
Pages: 56 to 62
Evaluation of the Digisonic® SP cochlear implant: patient outcomes and fixation system with titanium screws
Author(s): Guilherme Machado de Carvalho1; Alexandre Caixeta Guimarães2; Fabiana Danieli3; Lúcia Cristina Beltrame Onuki4; Jorge Rizzato Paschoal5; Walter Adriano Bianchini6; Arthur Menino Castilho7
DOI: 10.5935/1808-8694.20120034
Keywords: cochlear implantation, deafness, electrodes, implanted, hearing loss sensorineural, prostheses and implants.
Abstract:
Cochlear implants have revolutionized the way patients affected by severe hearing loss experience the world. Neurelec developed a fixation system with two titanium screws that requires no skull bone drilling.
OBJECTIVE: To describe the outcomes and procedure-related details of a series of patients implanted with the Digisonic® SP cochlear implant.
METHOD: This retrospective study analyzed patients submitted to cochlear implant placement within a period of 18 months. All patients had postlingual hearing impairment. Data was collected from patient charts and standard questionnaires answered by the surgeons in charge of carrying out the procedures.
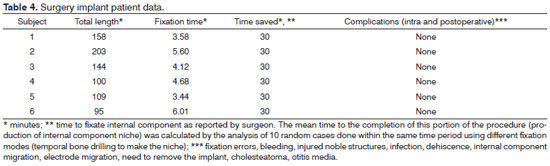
RESULTS: The six patients offered the Digisonic® SP cochlear implants were operated by experienced surgeons. The procedures took 95 to 203 minutes (mean = 135') to be completed, which is less time than what has been described for other fixation approaches. No complications were recorded and hearing improvement was satisfactory.
CONCLUSION: The Digisonic® SP cochlear implant developed by Neurelec offered good audiological results for adult patients, shorter surgery time, and no surgical or postoperative complications.
![]()
INTRODUCTION
Cochlear implants have revolutionized the way patients affected by severe hearing loss experience the world. Good outcomes with improved speech perception in patients of all age ranges have been observed1-3. Cochlear implants improve patient quality-of-life even more significantly in subjects aged 65 years and older4.
The implantation procedure is safe and reliable, but complications occur in about 16% of the patients. The most frequent adverse event relates to the insertion of electrodes into the cochlea, seen in about 4% of the cases5.
Migration of internal components has been described by many authors and may lead to cochlear implant malfunction and local infection6-8. Various implantation procedures have been described in the literature, with less invasive approaches gaining significant attention9-11.
The production of the bed on which the internal component is positioned is an important and onerous stage in the procedure, and has been the target of significant investment by cochlear implant makers12,13. More is thus spent in the development of surgical instruments and new materials such as titanium plates, propylene mesh, GORE-TEX, absorbable materials, and others14-16.
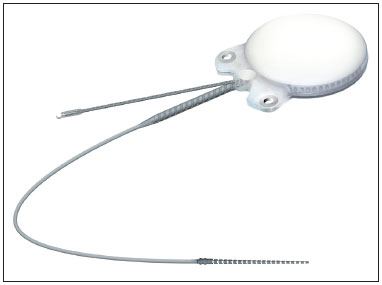
In regards to internal component fixation, Neurelec Inc. (Sophia-Antipolis, France) developed a fixation system with two titanium screws that requires no skull bone drilling17 (Figure 1).
Figure 1. Internal component with receptor, stimulator, and magnet, in a small sealed ceramic framework on a titanium base plate. Lateral niche has two fixation screws - Digisonic® SP developed by Neurelec.
Therefore, this study aimed to describe the outcomes and procedure-related details of a series of patients implanted with the Digisonic® SP cochlear implant and discuss the postoperative clinical and audiological findings observed in the last 18 months.
METHOD
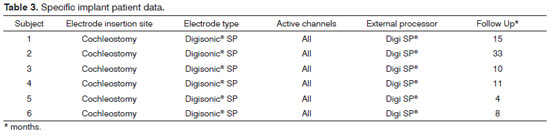
This retrospective study was carried out in a specialized tertiary care hospital. Patients implanted with Digisonic® SP cochlear devices were assessed for a period of 18 months.
A digital data collection protocol was designed. The following parameters were analyzed: age, gender, hearing loss etiology, time with hypacusis, side of implantation, pre and postoperative audiometric data (audiometry and speech perception testing), length of surgery, time to fixate the internal component, surgery complications, follow-up time in months. All patients in this series had post-lingual hearing loss (hearing was lost after they had developed speech and language skills).
Data was collected from patient charts, and a standard questionnaire answered by the surgeons who carried out the procedures.
The identity of the patients was not disclosed, as required by the ethical principles of the institution in which the study was conducted.
The patients
The patients selected for the study had Digisonic® SP cochlear devices implanted within a period of 18 months. All subjects followed a preoperative protocol in which the etiology of the impairment was investigated through lab workup, genetic tests, CT and MRI scans of the ears and mastoids, psychological evaluation, and thorough speech and hearing assessment.
The device
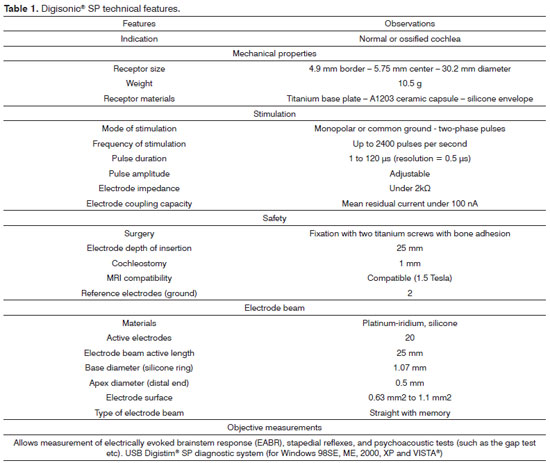
The Digisonic® SP cochlear implant system - made up by cochlear implant Digisonic® SP and speech processors Digi SP or Digi SP'K - was launched by French company Neurelec S.A. in 2004. This device belongs to the latest generation of implantable components developed by Neurelec and offers several advancements in relation to previous generation devices. The device's main features include an increased number of electrodes in the beam to allow for a greater number of active channels for stimulation and better spectral representation inside the cochlea, and a fixation system for the receiver-stimulator that uses two titanium screws and raises the stimulation rate through the "Mean Peak Interleaved Sampling" (MPIS) sound processing strategy.
Device internal component
Internal component Digisonic® SP developed by Neurelec is shown in Figure 1.
The receiver-stimulator (RS) is made up of a convex ceramic capsule, a titanium base plate, both coated with biocompatible silicone. Digisonic® SP is extremely compact, and both its electronic components used in signal decoding and its internal magnet are comprehended by one single structure with a diameter of 30 mm called the monoblock.
Table 1 describes the characteristics of Digisosnic® SP.
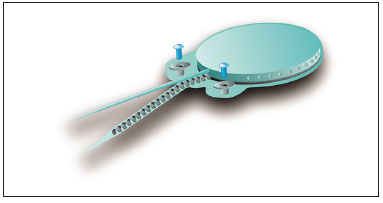
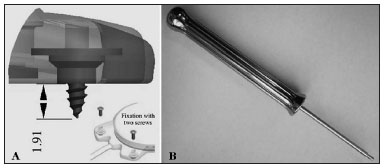
The device's fixation is done by two 3.4 mm titanium screws bolted into two small silicone-coated titanium orifices with 5 mm in diameter positioned in the ends of the receiver-stimulator, as shown in Figures 2 and 3. The screws are driven 1.91 mm into bone tissue17.
Figure 2. Digisonic® SP fixation system.
Figure 3. Digisonic® SP fixation system with two titanium screws driven 1.91 mm into the temporal bone. There is no need to drill or prepare the site. Developed by Neurelec S.A.
The compact structure and the fixation screws of the Digisonic® SP allow for quicker, less invasive surgery without the need to drill or suture bone tissue to position or fixate the implant17.
Digisonic® SP has atraumatic flexible screws that adjust quickly to the site in which they were positioned and connect firmly to the RS. The beam is made of 20 platinum-iridium electrodes, which allow the stimulation of up to 20 channels along the cochlea, with an active length of 25 mm and a stimulation rate of up to 1,000 pulses per second for each stimulation channel enabled by processing strategy MPIS. It is also equipped with silicone rings to facilitate insertion18.
The internal device contains a two-way telemetry system to record electrode impedance. Electrode impedance is recorded through diagnostic interface Digistim SP and software program Digistim for Windows SP® version 1.9.15, in which it is also possible to perform other objective measurements such as electrically evoked auditory brainstem responses (EABR) and electrically evoked stapedius reflex thresholds (ESRT)18.
EABR measurement is carried out routinely during surgery to verify device function and the stimulation of peripheral auditory nerve fibers19. In addition to that, EABR can also be used to predict the psychophysical levels to program the speech processor20, a particularly important step in the treatment of pediatric patients. EABR is measured at the end of surgery with the patient still under general anesthesia.
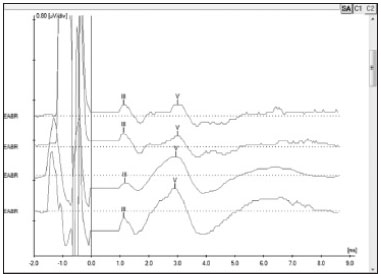
The procedure comprehends the electrical stimulation of nerve fibers through the electrodes inserted in the cochlea and the acquisition of responses through a conventional BAEP measurement device and a synchronization cord. It is possible to view waves II, III, and V, the last two being the most commonly observed; wave V is indicative of effective cochlear nerve stimulation. The absolute latencies of these waves are reduced when compared to conventional BAEP, given that the stimulation on EABR is performed directly in the cochlear nerve through the electrodes in the implant beam20 (Figure 4).
Figure 4. Electrically evoked brainstem responses measured during surgery for subject 5 electrodes 19, 14, 9 and 6 using Interface Navigator Pro and Software AEP version 7.0.0, Biologic. Waves III and V can be seen, showing proper nerve fiber stimulation.
Patients using the Digisonic® SP implant can undergo magnetic resonance imaging in scanners of up to 1.5 Tesla, if recommendations are followed18.
RESULTS
Table 1 shows the characteristics and technical details of the device used in the described procedures.
Tables 2 and 3 show general and specific data of the patients implanted with Digisonic® SP.
Table 4 shows the data on surgery-related occurrences and length of the procedure.
MRI and CT scans did not reveal radiological alterations.
EABR and ERST measurements were performed in all procedures at the end of surgery with the patients still under general anesthesia. Measurement outcomes were satisfactory and showed the device was functioning properly.
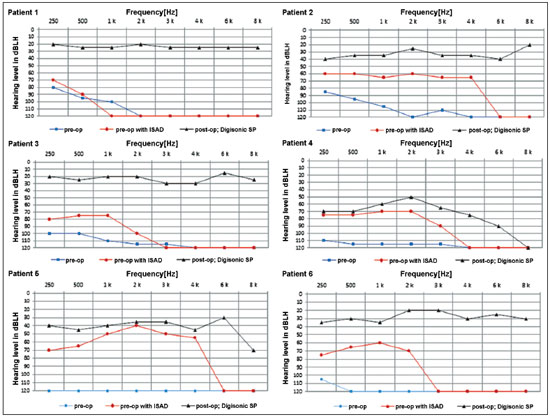
The graphs below show the pre and postoperative audiometric test results (Figure 5).
Figure 5. Pre and postoperative audiometric thresholds of six cochlear device implantation patients using Digisonic® SP. Note threshold improvement.
DISCUSSION
The Digisonic® SP cochlear implant developed by Neurelec has a fixation system with two titanium screws, which does not require the production of a niche to place the cochlear implant internal component or any drilling on the patient's skull bone. In addition to reducing the risks and complications associated with the production of the cochlear implant niche, this fixation system reduces the length of surgery.
In our study, six patients were implanted with Digisonic® SP by experienced surgeons, and surgery length ranged from 95 and 203 minutes, with a mean length of 135 minutes. The mean length of a conventional cochlear device implantation procedure is 255 minutes in the hands of experienced surgeons using the S-shaped retroauricular incision to make the implant niche, and 200 minutes when using a small retroauricular incision and a subperiosteal pouch21. Therefore, length of surgery in our series was shorter than that of procedures using other modes of fixation.
Studies looking into the cost of cochlear implants use a wide array of methods, and cost estimates may vary significantly depending on what is considered as part of the cost22. However, according to the literature, the cost of a unilateral implant in a patient with post-lingual hearing loss ranges between ? 30,026 (USD 21,018) and ? 45,770 (USD 32,039), with the device accounting for most of the cost23. In Brazil, the cost of a Digisonic® SP is similar to the cost of a conventional fixation device.
Despite the high cost, the benefits of cochlear implants far outweigh the costs as they enable hearing rehabilitation, improved communication, and better quality-of-life for deaf patients, with even more evident results seen in younger patients23-25.
The patients in our series had no complications, and the level of auditory gain was satisfactory in all cases. In general terms, mean complication rates of 16% have been described in cochlear device implantation procedures5. Despite the small size of our sample, our results were better than average.
CONCLUSION
The Digisonic® SP cochlear implant developed by Neurelec presented good audiological outcomes in our series, shorter length of surgery, and no intra or postoperative complications.
REFERENCES
1. Yang WS, Moon IS, Kim HN, Lee WS, Lee SE, Choi JY. Delayed cochlear implantation in adults with prelingual severe-to-profound hearing loss. Otol Neurotol. 2011;32(2):223-8.
2. Budenz CL, Cosetti MK, Coelho DH, Birenbaum B, Babb J, Waltzman SB, et al. The effects of cochlear implantation on speech perception in older adults. J Am Geriatr Soc. 2011;59(3):446-53.
3. Klop WM, Briaire JJ, Stiggelbout AM, Frijns JH. Cochlear implant outcomes and quality of life in adults with prelingual deafness. Laryngoscope. 2007;117(11):1982-7.
4. Chung J, Chueng K, Shipp D, Friesen L, Chen JM, Nedzelski JM, et al. Unilateral multi-channel cochlear implantation results in significant improvement in quality of life. Otol Neurotol. 2012;33(4):566-71.
5. Brito R, Monteiro TA, Leal AF, Tsuji RK, Pinna MH, Bento RF. Surgical complications in 550 consecutive cochlear implantation. Braz J Otorhinolaryngol. 2012;78(3):80-5.
6. Yun JM, Colburn MW, Antonelli PJ. Cochlear implant magnet displacement with minor head trauma. Otolaryngol Head Neck Surg. 2005;133(2):275-7.
7. Hoffman RA, Cohen NL. Complications of cochlear implant surgery. Ann Otol Rhinol Laryngol Suppl. 1995;166:420-2.
8. Migirov L, Muchnik C, Kaplan-Neeman R, Kronenberg J. Surgical and medical complications in paediatric cochlear im- plantation: a review of 300 cases. Cochlear Implants Int. 2006;7(4):194-201.
9. Mann WJ, Gosepath J. Technical note: minimal access surgery for cochlear implantation with MED-EL devices. ORL J Otorhinolaryngol Relat Spec. 2006;68(5):270-2.
10. Loh C, Jiang D, Dezso A, Fitzgerald O'Connor A. Non-sutured fixation of cochlear implants using a minimally-invasive approach. Clin Otolaryngol. 2008;33(3):259-61.
11. Carvalho GM, Valente JPP, Duarte ASM, Muranaka EB, Guimarães AC, Soki MN, et al. Electro acoustic stimulation of the auditory system: Unicamp's surgical approach. Braz J Otorhinolaryngol. 2012;78(1):43-50.
12. James AL, Papsin BC. Device fixation and small incision access for pediatric cochlear implants. Int J Pediatr Otorhinolaryngol. 2004;68(8):1017-22.
13. Dalchow CV, Werner JA. A new instrument for minimal access surgery in cochlear implantation. Otol Neurotol. 2005;26(4):678-9.
14. Lee DJ, Driver M. Cochlear implant fixation using titanium screws. Laryngoscope. 2005;115(5):910-1.
15. Davis BM, Labadie RF, McMenomey SO, Haynes DS. Cochlear implant fixation using polypropylene mesh and titanium screws. Laryngoscope. 2004;114(12):2116-8.
16. Otto RA, Lane AP, Carrasco VN. A new technique for securing cochlear implants. Otolaryngol Head Neck Surg. 1999;120(6):897-8.
17. Guevara N, Sterkers O, Bébéar JP, Meller R, Magnan J, Mosnier I, et al. Multicenter evaluation of the Digisonic SP cochlear implant fixation system with titanium screws in 156 patients. Ann Otol Rhinol Laryngol. 2010;119(8):501-5.
18. Vincent C, Ruzza I, Vaneecloo FM, Dubrulle F. Magnetic resonance imaging with the Digisonic SP Neurelec cochlear implant. Eur Arch Otorhinolaryngol. 2008;265(9):1043-6.
19. McCormick B, Archbold S (eds). Cochlear implants for young children: the Nottingham approach to assessment and rehabilitation. 2nd ed. London: Whurr; 2003. p.162-215.
20. Truy E, Gallego S, Chanal JM, Collet L, Morgon A. Correlation between electrical auditory brainstem response and perceptual thresholds in Digisonic cochlear implant users. Laryngoscope. 1998;108(4 Pt 1):554-9.
21. Prager JD, Neidich MJ, Perkins JN, Meinzen-Derr J, Greinwald JH Jr. Minimal access and standard cochlear implantation: A comparative study. Int J Pediatr Otorhinolaryngol. 2012;76(8):1102-6.
22. Nadège C, Valérie G, Laura F, Hélène DB, Vanina B, Olivier D, et al. The cost of cochlear implantation: a review of methodological considerations. Int J Otolaryngol. 2011;2011:210838.
23. Turchetti G, Bellelli S, Palla I, Berrettini S. Systematic review of the scientific literature on the economic evaluation of cochlear implants in adult patients. Acta Otorhinolaryngol Ital. 2011;31(5):319-27.
24. Turchetti G, Bellelli S, Palla I, Forli F. Systematic review of the scientific literature on the economic evaluation of cochlear implants in paediatric patients. Acta Otorhinolaryngol Ital. 2011;31(5):311-8.
25. Peñaranda A, Mendieta JC, Perdomo JA, Aparicio ML, Marín LM, García JM, et al. Economic benefits of the cochlear implant for treating profound sensorineural hearing loss. Rev Panam Salud Publica. 2012;31(4):325-31.
1. MSc in Medicine, MD, ENT (Fellow in Otology. UNICAMP).
2. MD. (ENT Medical Resident. UNICAMP).
3. MSc in Bioengineering. (Specialist in Cochlear Implants).
4. Speech and Hearing Therapist. (Specialist in Cochlear Implants. UNICAMP).
5. MD, PhD, ENT. (Professor of Otorhinolaryngology. Head of the Otology Service. UNICAMP).
6. MSc in Medicine. MD, ENT. (Head and Coordinator of the Otology, Audiology, Cochlear Implant, and Implantable Hearing Aid Service. UNICAMP).
7. MD, PhD, ENT. (Head and Coordinator of the Otology, Audiology, Cochlear Implant, and Implantable Hearing Aid Service. UNICAMP).
Otology, Audiology and Implantable Ear Prostheses Ear, Nose, Throat and Head & Neck Surgery Department. E-mail: otorrino@fcm.unicamp.br
Send correspondence to:
Dr. Guilherme Machado de Carvalho
Disciplina de Otorrinolaringologia - UNICAMP. Faculdade de Ciências Médicas. Universidade de Campinas - UNICAMP
Campinas - SP. Brasil. CEP: 13083-970
Caixa Postal: 6111
Tel: +55 (19) 3521-7523. Fax: +55 (19) 3521-7563
E-mail: guimachadocarvalho@gmail.com
Paper submitted to the BJORL-SGP (Publishing Management System - Brazilian Journal of Otorhinolaryngology) on August 6, 2012.
Accepted on October 2, 2012. cod. 9938.