Year: 2002 Vol. 68 Ed. 4 - (3º)
Artigo Original
Pages: 462 to 466
Auditory central avaliation in Parkinson Disease with BERA and P300
Author(s):
José C.A. Pineroli(1),
Danielle S. de Campos(2),
Gislaine R.Wiemes(3),
Murilo S. Meneses(4),
Marcos Mocellin(5)
Keywords: Parkinson disease, event-related potentials, P300, evoked potential, auditory, brain stem
Abstract:
Introduction: The Parkinson Disease (PD) is one of the disturbance of movement, that attain elderly people. It promotes the progressive and irreversible loss of cells of black substance (locus niger) from the mesencephalon, as a result it will have a reduction of dopamine production leading the syntomatology of PD. Aim: Analyzing the electrophysiologic tests, able to control the functional integrity of the cerebral ways, in the diagnosis and/or prognosis of patients with PD, once these not-invasive tests of easy management, fast and simple understanding, whom have participation in the management. Study form: Random clinical prospective. Material and method: group of 32 patients with previous diagnosis of PD, undertaken to the evoked potential Bera and P300. Male and female patients, among 44 and 85 years old, time of disease variation from 8 to 21 years old of evolution. Results: The Bera and P 300 values found in the patients with PD will not indicate different limits of normality to the age. Conclusion: it was noticed integrity of auditive ways in patients with PD. Once the latency of P300 rises in a linear form with the age after 45 years, accepting a rise of 1-1,5ms a year1; it was observed integrity in the cerebral ways to reflect the ability of cognitive performance in patients with PD without important dementing compromise.
![]()
Introduction
Parkinson's disease (PD) is a common disease with prevalence of 1 to 2 cases by 1,000 in the general population and 1 in 100 among people over 65 years2. The progression consists of loss of nigra substance cells in the midbrain, in an irreversible and disabling fashion, compromising cognitive and intellectual functions. Since it is a chronic progressive disorder, early diagnosis and immediate administration of neuroprotective medication can improve prognosis of these patients3. Therefore, we tried to study the value of electrophysiologic tests in the diagnosis of PD. The tests, although they do not indicate the pathological basis of the cerebral damage, can monitor the functional integrity of cerebral pathways4. Two of the tests were used: brainstem auditory evoked potential - BERA e long-latency or cognitive auditory potential, known as P300.
Material and Method
Thirty-two patients with diagnosis of PD at the Ambulatory of Neurology, Hospital de Clinicas HC-UFPR, participated voluntarily in the study. The diagnosis was confirmed by a neurologist in the presence of at least two of the four cardinal signs and symptoms of the disease - rest tremor, muscle rigidity, bradykinesia and posture abnormalities5. We selected patients with PD without evident dementia impairment. The patients signed an Informed Consent Term and were referred to the Department of Otorhinolaryngology, HC-UFPR, to undergo potential evoked tests of BERA and P300. After the history, we performed inspection of the auditory canal and pure tone and vocal audiometry. To conduct the present study, we used secondary data from a research study named "Central Auditory Assessment with BERA and P300 in Patients with PD" (sponsored by CNPq), and previously approved by the Medical Ethics Committee at HC-UFPR.
BERA was performed as follows:
· in a sound-proof booth;
· clicks were used as stimuli, whose sound energy was more concentrated in the range 2K to 4K Hz;
· as to electrode placement, the active one was placed on the forehead, the reference on the earlobe and the mass on the opposite earlobe. The skin had been previously cleaned with NuprepÒ paste and we applied TEM-20Ò conductive paste between the skin and the electrodes, to allow better conductivity of electrical current, fixed with micropore tape;
· stimuli were presented ipsilaterally, that is, the stimulus was presented first to the ear that had the reference electrode; for example, right ipsilateral: phone with the sound stimulus - right ear (RE), reference electrode - right earlobe, mass electrode - left earlobe;
· once the electrodes were placed, we adjusted the earphones. We selected stimuli and number of presentations. The number of stimuli was 20 clicks per second. The test was started with 40 to 50dB SL above the expected thresholds, so as to determine the response profile. When the curve was not defined, intensity was increased;
· once the response was recorded, the curve was stored in the equipment memory and printed. The response level was studied by decreasing sound intensity from 10 to 20dB, successively. We observed latency increment in different complexes (I to V);
· minimum response level was defined as absence of analyzable response in wave V prompted by low intensity stimuli;
· we masked the contralateral ear with white noise upon click stimuli;
· the equipment used for the test was Amplaid MK-12 Monitoring;
· we recognized 3 constant waves: I, III and V. The tracing was considerably affected by intensity of stimulation. The different waves were less identifiable as the stimulus intensity was progressively decreased, except for wave V, in which the levels were recognized close to the psychoacoustic thresholds.
· the reference used as absolute latency of waves was: I- 1.5 to 1.8ms; III- 3,5 to 3.8ms; V- 5.5 to 6.0ms. Relative latency admitted between waves I, III and V was 2 ms.
To assess cognitive evoked potential P300 we required: silent and semi-dark room, reclining chair with head support, silver chlorite electrode, including grounding electrode - with acoustic impedance below 5kohms on each side; electroencephalogram amplified 10,000 times with 1 to 30Hz band and sensitivity of about 50 microvolts. Artifacts were rejected using the internal circuit of the equipment that excluded from the measurement process all recordings of potential in excess of 45 microvolts.
All patients were instructed to attend to 2 tones and to respond only to rare stimuli (2000Hz - denominated rare stimuli - ER), whereas the series of frequent sounds (750Hz - designated frequent stimuli - EF) were presented, through earphones, at regular intervals of 800 milliseconds, at 4/1 proportion, both at 70dB HL (70 decibel hearing level intensity). It is important to point out that stimuli were presented binaurally. Responses to frequent and rare stimuli were measured separated.
During the test, we presented approximately 300 stimuli (80% EF and 20% ER) and the patients had to demonstrate tone discrimination by counting ER in loud voice, for example. The procedure lasted on average 40 minutes, being 15 to place the electrodes on the patient, data inputting and checking of the appropriate impedance. Other 25 minutes were dedicated to performing the test per se.
Electrodes were connected on the following sites: forehead (ground), vertex (Cz) - both on the midline, and ipsilateral (A1) and contralateral (A2) earlobes. We had previously cleaned the skin with NuprepÒ paste and used conductive paste TEM-20 between the skin and the electrodes to better improve conductivity of electrical current, using fixation with micropore tape. Electrodes were connected to a pre-amplifier fixed to the patient's clothes. The patient was instructed to remain alert and relaxed to perform the test. Before the test began, earphones were positioned (blue phone on the left ear and red phone on the right ear), and the patient was questioned about the comfort of both phones and chair.
After triggering EF and ER sound stimuli, the waveforms were recorded6 and subcomponents were divided into:
- N1 (P100) - maximum negativity potential field, peak at 70 to 120ms after EF, with negative polarity;
- P2 (P200) - maximum positivity potential field, peak at 104 to 230 ms after EF, with positive polarity;
- N2 (N200) - component of negative potential, peak at about 206ms after ER, with negative polarity;
- P3 (P300) - component of maximum positive potential, peak at 256 and 500ms after ER and P2, with positive polarity. For the test to be considered normal, wave latency should not have been in excess of 400ms, plus age correction.
We measured latency of waves N1, N2, P2 and P3 that comprise the P300 tests, recorded in the memory of the equipment AMPLAID MK-12 Monitoring and printed for later analysis. The studied wave was positioned after N2 and the latency was measured at P3 peak. Latency measures derived from tracings of Cz, A1 and A2, in order to define the components of P300. The search did not take into account wave amplitude of P300 wave, owing to the fact that its diagnostic importance is still unknown because of great variation in values7.
Results and Discussion
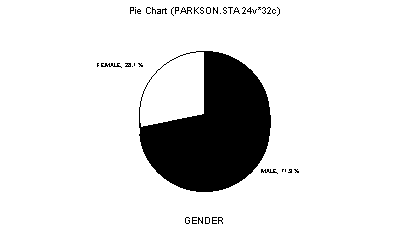
Figure 1. Distribution of subjects according to gender.
Figure 2. Mean values of P3 wave -RE (ms) at P300.
Figure 3. Mean values of P3 wave-LE (ms) at P300.
The population consisted of people aged from 44 to 85 years, mean age of 64 years (Table 1), compatible with the literature that reported the highest prevalence of PD between the elderly, with onset of affection normally between 50 and 70 years3. Among the subjects studied in the sample, 23 were men (71.89%) and 9 were women (28.1%) (Figure 1), despite the fact that the disease has even distribution between genders2.
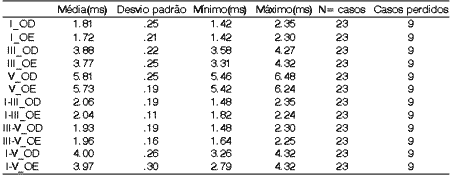
In twenty-three patients, BERA presented mean latency values within the normal ranges, that is I- 1.5 to 1.8ms; III- 3.5 to 3.8ms; V- 5.5 to 6.0ms8. Relative latency advocated for waves I, III and V is 2ms, but in our sample, we found the following means for interval I-III: 2.06ms and 2.04ms on the right and left ears, respectively (Table 2). However, a value up to 2.20ms can be accepted as normal, considering the age of patients. Once there was significant interference in the recording of BERA in 9 of the 32 patients, such values were not considered in the final analysis.
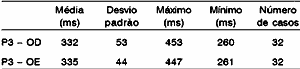
As to P300, all patients had P3 waves recorded on both sides. Values ranged from 260 to 453 ms on the right and 261 to 447 on the left ear. P3 wave measures ranged from 332ms to 335ms on the right and left ears, respectively (Table 3, Figures 2 and 3). Accepting as normal to have 1 to 1.5 ms added per year to P3 latency values after the age of 45 years1, the values of the sample were within the expected spectrum for the age, except for one single value that exceeded 2ms of what was expected. We observed during the P300 test that it was difficult to perform the appropriate wave tracing (high rejection).
The purpose of the present study was to demonstrate the feasibility to use electrophysiologic tests to assess cerebral pathways concerning cognitive functions. Our next study will address clinical neurocognitive assessment of patients to compare the results to those of electrophysiologic tests.
Table 1. Distribution of subjects according to age (years).
Table 2. Mean values of BERA.
Table 3. Mean values of P3 wave at P300.
Conclusion
BERA results did not present evident abnormalities between patients with PD when compared to reference values proposed by the literature for subjects without disease. It was inferred that progressive neuronal loss of midbrain nigra substance caused by PD does not impair auditory sensitivity nor the integrity of auditory pathways measured by BERA (from auditory nerves, passing through cochlear nuclei, olivary superior complex, lateral lemniscus, and inferior colliculus). As to P300 results, they were within the normal range when compared to the literature, and we inferred that PD does not cause loss of cognitive performance in all cases. We would like to emphasize the need to have a control group assessed with the same technique and by the same professionals, as well as a neurocognitive assessment of patients with PD, to come to more accurate conclusions about the value of electrophysiologic tests in PD.
References
1. Picton TW, Stuss DT, Champagne SC, Nelson RF. The effects of age on human event-related potentials. Psychophysiology 1984;21(3):312-325.
2. Harrison TR. Medicina Interna. 14ª Edição. Editora Mc Graw Hill; 1998. Capítulo 361, p.2424-5.
3. Meneses MS, Arruda WO, Hunhevicz SC, Ramina R, Pedrozo AA, Tsubouchi MH. Comparison of MRI-guided and ventriculography-based stereotactic surgery for Parkinson's disease. Arquivos de Neuro Psiquiatria 1997;55(3B):547-552.
4. Wood CC, Allison T, Goff WR, Williamson PD, Spencer DD. On the neural origin of p300 in man. Prog brain Res 1980;54:51-6.
5. Ward CD, Gibb WR. Research criteria for Parkinson's disease. In: Streifer MB, Korczyn AD, Melamed E, Youdim MBH (eds.). Advances in Neurology. New York: Raven Press; 1990. p. 245-249.
6. Fukuda Y. P300 - Um potencial cerebral relacionado a eventos. Revista Brasileira de Atualização em ORL 1994;1(3):196-8.
7. Pfefferbaum A, Ford JM, Wenegrat BG, Roth WT, Kopell BS. Clinical application of the P3 component of event-related potentials. I. Normal aging. Eletroenceph Clin Neurophysiol 1984;59:85-103.
8. Costa FºAO, Celani AC. Audiometria de respostas elétricas do tronco cerebral. A Folha Médica 1993;107(1):43-48.
9. Antal A, Pfeiffer R, Bodis WI. Simultaneously evoked primary and cognitive visual evoked potentials distinguish younger and older patients with Parkinson's disease. J Neural Transm 1996;103(8-9):10053-67.
10. Aotsuka A, Weate SJ, Drake ME, Paulson GW. Event-related potentials in Parkinson's disease. Electromyogr Clin Neurophysiol 1996;36(4):215-20.
11. Bodis WI, Borod JC. Modality dependent changes in event-related potentials correlate with specific cognitive functions in nondemented patients with Parkinson's disease. J Neural Transm Dis Dement Sect 1995;9(2-3):197-209.
12. César CPHR & Munhoz MSL. Avaliação dos potenciais de longa latência relacionados a eventos em indivíduos jovens e adultos saudáveis. Acta Awho 1997;16(3):114-122.
13. Chia LG, Cheng LJ, Chuo LJ, Cheng FC, Cu JS. Studies of dementia, depression, electrophysiology and cerebrospinal fluid monoamine metabolites in patients with Parkinson's disease. J Neurol Sci 1995;133(1-2):73-8.
14. Diniz J Jr. Contribuição ao estudo potencial evocado auditivo de longa latência em crianças. São Paulo, 1996. Tese de Mestrado - Universidade Federal de São Paulo - Escola Paulista de Medicina.
15. Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson's disease: evidence supporting it. Ann Neural 1992;32:804-812.
16. Fahn S, Elton RL. Members of the UPDRS Development Committee. Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M (eds.). Recent Developments in Parkinson's Disease. Vol. 2. Florham Park, NJ: Macmillan Health Care Information; 1987. p. 153-164.
17. Goodin DS, Squires KC, Henderson BH & Starr A. Age-related variations in evoked potentials to auditory stimuli in normal human subjects. Electroencephalography and Clinical Neurophysiology 1978;44:447-58.
18. Hoehn MM, Yahr MD. Parkinsonism: Onset, progression and mortality. Neurology 1967;17:427-442.
19. Lagopoulos J, Clouston P, Barhamali H, Gordon E, Li WM, Lesley J Morris JG. Late components of event-related potentials and their topography in Parkinson's disease. Mov Disord 1998;13(2):262-7.
20. Lagopoulos J, Clouston P, Barhamali H, Gordon E, Lim CL, WM, Lesley J, Morris JG. Dysfunctions of automatic (P300a) and controlled (P 300b) processing in Parkinson's disease. Neurol Res 1998;20(1):5-10.
21. Marseillan RF, Oliveira JAA, Del Vecchio F. Audiometria de respostas elétricas do tronco cerebral humano. (BERA). Res Bras Otorrinolaringol 1977;43:29.
22. Martinez MP. Rating scales in Parkinson's disease. In: Jankovic J, Tolosa E. Parkinson's disease and Movement Disorders. 2ª ed. Baltimore: Williams and Wilkins; 1993. chapter 17, p. 281-292.
23. Meneses MS, Teive HAG. Doença de Parkinson. 1ª ed. Guanabara Koogan; 1996. Capítulo 1, 3, 5-7, 16.
24. Ohlrich ES, Barnet AB. Auditory evoked responses during the first year of life. Eletroenceph Clin Neurophysiol 1972;32(2):161-9.
25. Parkinson Study Group. Impact of deprenyl and tocopherol treatment on Parkinson's disease in DATATOP subjects not requiring levodopa. Ann Neurol 1996;39:29.
26. Polich J, Ladish C Bloom FE. P.300 assessment of early Alzheimer's disease. Electroenceph Clin Neurophysiol 1990;77(3):179-89.
27. Sohn YH, Kim GW, Huh K, Kim JS. Dopaminergic influences on the P300 abnormality in Parkinson's disease. J Neurol Sci 1998;158(1):83-7.
28. Stanzione P, Semprini R, Pierantozzi M, Santilli Am, Fada L, Traversa R, Peppe A, Bernardi G. Age and stage dependency of P300 latency alterations in non-demented Parkinson's disease patients without therapy. Eletroenceph Clin Neurophysiol 1998;108(1):80-91.
29. Verleger R, Neukäter W, Kömpf D, Vieregge P. The reasons for the delay of P3 latency in healthy elderly subjects. Eletroenceph Clin Neurophysiol 1991;79:488-502.
30. Yahr MD, Duvoisin RC, Barrett RE, Hoehn MN. Treatment of Parkinsonism with levodopa. Arch Neurol 1969;21:343-354.
[1] Physician, Service of Otorhinolaryngology, In Charge of the Division of Auditory Electrophysiology, Federal University of Parana -UFPR.
[2] Undergraduate, Medical School, Federal University of Parana-UFPR, scholarship by CNPq.
[3] Speech and Language Pathologist and Audiologist, Service of Audiology and Auditory Electrophysiology, Service of Otorhinolaryngology, Federal University of Parana-UFPR.
[4] Neurosurgeon, Professor and Head of the Discipline of Neuroanatomy, Federal University of Parana -UFPR.
[5] Faculty Professor and Head of the Discipline of Otorhinolaryngology, Federal University of Parana -UFPR.
Study conducted in the Service of Otorhinolaryngology, Hospital de Clínicas HC-UFPR
Address correspondence to: José Carlos A. Pineroli - Av. Getúlio Vargas, 2151
80250-180 - Curitiba - Parana - Tel/Fax: (55 41) 242.3174
E-mail: jcpineroli@uol.com.br daniscam@bol.com.br