Year: 2002 Vol. 68 Ed. 3 - (19º)
Artigo de Revisão
Pages: 417 to 423
Childhood hearing impairment
Author(s):
Pedro Oliveira(1),
Fernanda Castro(2),
Almeida Ribeiro(3)
Keywords: childhood hearing impairment, otoacoustic emissions, auditory brainstem responses, screening
Abstract:
Childhood Hearing Impairment is nowadays considered as a Health Care matter due to its high prevalence and to its multiple consequences. As a developing subject, frequent updates are justified to keep up with the evolution of techniques and knowledge. This paper aims to discuss the matter from a global point of view, paying particular attention to the Screening Models and Instruments available.
![]()
Introduction
Hearing impairment in children has a significant impact on the society from the economic perspective, involving high costs in detection and rehabilitation, and from the psychosocial perspective, affecting the subject, his family and the society as a whole. In fact, it interferes in the development of verbal and language skills, which leads to learning difficulties and negative impacts on social, emotional, cognitive and academic development. Therefore, it is essential to have early diagnosis so as to take the best of central nervous system plasticity at younger ages.
Bilateral profound deafness affects about 1 in 1,000 newborns, a figure that increases to 20 to 40 in 1,000 if we consider only the children in the Neonatal Intensive Care Unit (NICU). If we take into account also the less severe or unilateral cases (3 to 6 in 1,000) and hearing loss acquired in childhood years, we can realize the real magnitude of the problem.
Definition and Classification
Permanent bilateral hearing loss in children is defined as a bilateral hearing loss characterized by hearing thresholds higher than 40dB in the best ear, considering the frequencies of 500, 1000, 2000 and 4000 Hz, without hearing aids. It may be classified according to severity, onset and level of hearing loss.
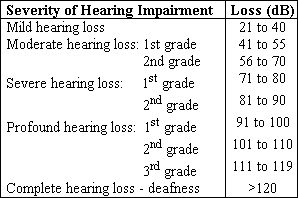
Concerning severity of hearing loss (Chart 1), first there are mild cases, in which words are detected by the child, but the phonemes are not. In turn, in cases of moderate hearing loss, comprehension thresholds and learning skills are beyond hearing thresholds, leading to a number of difficulties. In such situations, hearing aid fitting and speech therapy may provide near-normal learning. The same does not apply to severe or profound deafness, since words are not understood, requiring special education and hearing aid use.
As to onset of hearing loss, hearing losses are classified according to language acquisition and reading skills. Post-lingual deafness affects the child after he can read and speak, which is supported by the reading skills of the child. Peri-lingual deafness is noticed in children that speak but can not read, a situation that unless effectively treated, may quickly lead to language degeneration. Pre-lingual deafness is characterized by absence of auditory memory, which makes it extremely difficult to come up with language structure.
Finally, there is the classification of hearing loss based on level of lesion. Conductive or transmission hearing loss is a problem of insufficient intensity, not associated to distortion. It is an extremely frequent problem in children, ten times more frequent that sensorineural loss, and it normally does not cause losses below 60 dB, or in other words, does not go beyond the classification of moderate loss. The most common etiologies are acquired, especially serous-mucous otitis, cerumen block, foreign body and chronic otitis. As to congenital situations, they amount for less than 1% of the cases and they may indicate inner ear anomalies.
Perceptual or sensorineural hearing loss is a result of cochlear or retrocochlear damage and it is associated with hearing perception distortions that are difficult to compensate. As a consequence, it is a frequent cause of profound loss, requiring specific rehabilitation and the earlier it starts, the more successful it may be. This type of hearing loss is normally divided into genetic and non-genetic. The genetic causes are responsible for one third to half of the cases and according to onset, they may be congenital (pre-natal) or post-natal (Chart 2). Conversely, the situations of non-genetic cause comprise pre-natal, peri-natal and post-natal affections (Chart 3). There are still 2 to 30% of cases in which it is not possible to include sensorineural loss into any of the categories, being then classified as sensorineural hearing loss of unknown etiology.
Finally, mixed hearing loss in children is present when there are both conduction and perception components. These are situations that require attention because the transmission component aggravates the sensorineural impairment, reason why these affections should be systematically investigated and treated.
SCREENING
Deafness in children compromises permanently language acquisition and social, emotional and academic development of the subject, since the first 3 years are the most important ones for speech learning.
However, before the introduction of the screening programs in some states of the United States, the mean age at diagnosis of bilateral profound hearing loss was 18 months to 3 years, which is undoubtedly late. In fact, the Committee for Infant Hearing (1994) and the European Consensus of Milan (1998) defended diagnosis at 3 months of age and therapeutic intervention at 6 months, which justifies the need for screening.
It is a consensus today that simply observing the child and respecting parents' suspicion is not enough to detect deafness in the first year of life. Thus, since it was impossible to study all newborns, some criteria for high risk children were defined (Chart 4). We should, however, be aware that the attention should not be focused only on newborns, because approximately 10 to 20% of the children who develop profound hearing loss have its onset after 3 years of age.
This type of screening performed in high risk children is positive especially from an economic perspective, because it suggests the performance of tests in only 10% of the children. Thus, it is a useful tool if it is impossible to screen all babies and as a starting point for a more comprehensive plan. Its main disadvantage is that it detects only half of the significant cases.
This is the reason why the NIH Consensus Statement and the European Consensus of Milan had defined the objective of Universal Screening of newborns using quick, feasible and easy methods. In fact, hearing loss in children fulfills all the criteria to be submitted to universal screening programs, since it is an easily detectable disease by clinical parameters, there are easy to apply tests with high sensitivity and specificity, it is possible to provide therapeutic intervention, and early action provides better prognosis and good cost-effective ratios. Actually, hearing loss screening is cheaper than phenylketonuria, hypothyroidism and sickle cell anemia screening programs.
With this in mind, the Marion Downs National Center for Infant Hearing was created in the USA, with participation of 19 states and whose goal is to implement Universal Screening, amounting to 95% of the newborns.
screening methods
Generally speaking, the most important screening methods today are Behavioral Tests, Brainstem Auditory Evoked Potentials and Otoacoustic Emissions.
Behavioral Tests
Up to some time in the past, behavioral tests used for hearing screening and assessment were considered primitive and unreliable. However, the acquisition of new material and the use of new methods allow us to conclude that Behavioral Audiology is useful, especially when associated with electrophysiological methods.
Behavioral Observation Audiometry
This test is used especially between birth and 6 months of age and consists of free field stimulation through sound and loud intensity noise whose frequency is controlled by the examiner. The response may be sleep pattern alteration, or facial expression, Moro's reflex or cochleo-palpebral reflex. The main disadvantages of this group of tests that include Veit-Bizaguet method and Crib-o-Gram are no detection of unilateral or mild loss, likelihood of the patient getting habituated to the stimulus and the fact that it depends on the examiner and the wakefulness of the baby. This is the reason why it should not be employed without electrophysiological methods.
Visual Reinforcement Audiometry
It is used after 6 months of age, a time when the child has enough control of the neck and trunk and as of the moment it becomes possible to have stimulus-conditioned response. It consists of the use of visual stimuli, including lights or toys in movement as reinforcement to the response to the auditory stimulus.
Orientation Reflex Test - Investigation
These tests, which include Nome Test and Boel Test are essentially used between 6 months and 2 years of age. It consists of causing the reflex or the orientation movement of the child that searches for the sound with her head.
Conditioned Play Audiometry
It is a natural progression after visual reinforcement audiometry and it is normally employed in children used to the first test and in those older than 3 years. It consists of performing a simple task, such as placing an object in a basket, after listening to the auditory stimulus.
Play Audiometry (Peep-show)
Peep-show uses the same age group and the child informs he has listened by pressing a button that activates a device that gives the child a reward, such as a candy or piece of chocolate.
Audiometry in Sound Booth
To undergo this test, degrees of maturation and coordination are required, which are usually found at about 3 to 4 years of age. The method has the advantage of effectively assessing each ear separately. As to Vocal Audiometry, it is more reliable than pure tone audiometry in children below 5 years, allowing better exploration of the global perception and speech discrimination. Pure Tone Audiometry is normally employed after the age of 5, when children are then capable of responding to the test as adults.
Behavioral Methods should be performed in silent environments and if the child does not pass a test for her age, a less sophisticated one should be tried.
Generally speaking, the disadvantages of these tests are time spent with specialized and qualified professionals, need for appropriate facilities, difficulty to perform them with children who do not cooperate and low sensitivity to hearing loss screening. It is a current consensus that Behavioral Audiometry in younger ages has limited value, but it is also an important screening method after the neonatal period.
Auditory Brainstem Evoked Potentials (BERA)
Since the first description of auditory evoked potentials in 1971 by Jewett and Williston, its interest in ENT has grown, and it is considered by many as the most objective method to assess hearing in the newborn. It translates the activity of the auditory nerve up to the brainstem in response to auditory stimuli, which are normally called clicks for screening purposes and whose response reflect synchronous neural activation of the auditory pathway. The response is usually registered after 5 to 6ms, manifested by waves. Wave V, the most important one, enables definition of electrophysiological thresholds, which consists of minimal intensity capable of triggering the response. Based on this value, we can deduct the audiometric thresholds by reducing 20 dB. It is important to realize that wave latency and amplitude times in children below 18 months vary as a result of age and comparisons should be made by age range. As the age increases, latency reduces and amplitude increases, a fact that is probably due to progressive myelinization, increased axonal diameter and higher functionality of synapse.
BERA has some advantages compared to other screening methods: it is independent from voluntary response, non-invasive, highly reproducible and reliable, has high sensitivity and more specificity than otoacoustic emissions, leading to lower referral rates (3 to 5%) and few false-positive. In specific situations, such as hyperbillirubinemia, evoked potentials are also fundamental because the auditory pathway damage is retrocochlear, at the cochlear nucleus, and it may remain undetected in other tests, such as otoacoustic emissions. They are also equally important in the recently described auditory neuropathy, an affection characterized by normal otoacoustic emissions and absent or very abnormal BERA responses. As to disadvantages, we can list high cost and time spent, the need for qualified personnel and the capacity to assess only 1 to 4kHz frequencies, with deficit in high and low frequencies.
It is not an appropriate method for first line Universal Screening, and it is preferably used in high risk children, normally at the NICU, or in situations in which the child failed in the screening by other methods.
In order to compensate the disadvantages, a new type of automated evoked potential (or Automated BERA) was recently launched. It uses stimulus with acoustic spectrum between 750 and 5,000Hz, comprising noise reduction and myogenic activity devices, which allows testing of newborns undergoing oxygen therapy, and it has an easy method of algorithmic analysis that interprets the results as "pass/fail". It proved to be similar to classical BERA in 95% of the cases, and it was easy, quick and little influenced by middle ear effusion or cerumen built-up, which show it is a potential instrument to perform universal screening.
Chart 1. Classification of Pediatric Deafness According to Severity.
Chart 2. Sensorineural Hearing Loss from Genetic Causes.
Sensorineural Hearing Loss from Genetic Cause
Congenital:
Dysplasias (Michel, Mondini, Sheibe)
Congenital genetic SHL not associated to malformations
Congenital genetic SHL associated to malformations
Cranial dysmorphia (S. Crouzon)
Limb dysmorphia (S. Wildewanck)
Pigmentation anomalies (S. Waardenburg)
Ophthalmic affections (S. Usher, S. Halgren)
Cardiac diseases (S. Jervell and Lange-Nielsen)
Thyroid pathology (S. Pendred)
Chromosome abnormalities (trissomy and monossomy)
Syndrome of wide vestibular aqueduct
Congenital peri-lymphatic fistula
Post-natal:
Genetic post-natal SHL not associated to malformations
Genetic post-natal SHL associated to malformations:
Metabolic disease (mucopolysacharidosis)
Ophthalmologic malformations (S. Cockayne)
Renal disease (S. Alport, S. Fanconi)
Skeletal malformations (S. Klippel-Feil)
Neurological diseases (Neurofibromatosis)
Chart 3. Sensorineural Hearing loss from Non-genetic Causes.
Sensorineural Hearing loss from Non-genetic Causes
Pre-natal:
Ototoxic drugs used during pregnancy (aminoglycosides, diuretics, thalidomide, alcohol)
Congenital Infections (Rubella, CMV, other TORCH)
Other causes (1st trimester hemorrhage, vitamin deficiency, hormone therapy, pelvic irradiation)
Peri-natal:
Severe neo-natal jaundice
Low birth weight (<1,500 g) and Prematurity
Peri-natal asphyxia
Delivery trauma
Post-natal:
Infections (labyrinthitis, meningitis, parotiditis, measles,...)
Head Trauma
Acoustic Trauma
Ototoxic Drugs
Neoplasias (acoustic nerve neurinoma, leukemia)
Metabolic diseases (hypothyroidism, diabetes)
Auto-Immune diseases
Idiopathic sudden deafness
Chart 4. Risk Factors for Childhood Hearing Loss.
Risk factors for childhood hearing loss
Neonatal:
Family history of hearing loss
TORCH Congenital infections
Head and neck anatomical malformations
Low birth weight (<1,500 g)
Severe hyperbillirubinemia
Signs of syndromes associated to deafness
Maternal use of drugs or ototoxic medication
Peri-natal asphyxia with Apgar < 4
NICU hospitalization
Head trauma during delivery
During childhood (>3 months):
Bacterial meningitis, encephalitis or labyrinthitis
Peri-natal CMV
Acoustic Trauma
Head trauma
Ototoxic drugs
Family history
Repetitive otitis media with effusion
Chart 5. Physiological Mechanisms of Otoacoustic Emissions.
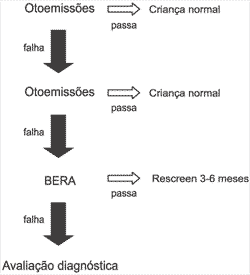
Chart 6. Model of Universal Screening (NIH).
Otoacoustic emissions (OAE)
The historical origin of otoacoustic emissions dates back from 1948, when Gold advocated that the cochlea was capable of great selectivity of frequency through mechanisms of existing active feedback with minimal energy expense. However, only 30 years later did David Kemp prove that the feedback mechanism was capable of producing low intensity sounds (10 to 15dB) recordable at the external auditory canal, which were named otoacoustic emissions. Kemp made a revolution to auditory physiology by demonstrating that there are active mechanisms and internal reflexes at the cochlea and he confirmed they were not linear but distorted, which are characteristics needed to detect low intensity sound and to differentiate sounds that have close frequency.
Currently, the origin of OAE has been well documented in movements of contraction/extension of outer hair cells of Corti's organ, including its amplification function (Chart 5).
The clinical importance of OAE is a result of the fact that most pathologies that lead to sensorineural loss, especially those associated with hearing loss in children, present outer hair cell affection as the first or secondary damage.
Otoacoustic emissions may be divided into spontaneous, when they are present without stimulation, and evoked, when they follow stimulation. The former is present in 60% of normal subjects, at 10 to 20dB intensity and frequency of 1 to 2kHz. Evoked otoacoustic emissions include Stimulus-frequency otoacoustic emissions, transient otoacoustic emissions and Distortion Product otoacoustic emissions.
Stimulus-frequency otoacoustic emissions are a result of stimulation with one continuous low intensity pure tone stimulus. Clinical acceptance is reduced because it requires special equipment to differentiate stimulus from the response, whose frequencies are very close.
Evoked transient otoacoustic emissions, the first ones described by Kemp, are the most frequently used because it is easy to perform the test and the necessary software is affordable. They are the result of transient acoustic stimulation of the whole cochlea with clicks produced by loudspeakers that are recorded by a microphone at the external auditory canal. The assessment is limited to frequencies below 5kHz. The presence of a positive response suggests that the hearing threshold is below 30dB, and there are different parameters used, depending on the author. It is an excellent screening method because it is quick, reliable, does not require patient's cooperation and is non-invasive.
Distortion product otoacoustic emissions are the consequence of simultaneous cochlear stimulation by two pure tone sounds with similar frequencies (F1 and F2), leading to overlapping of excitation pattern of basilar membrane, the region from where otoacoustic emissions originate. The most important distortion product is named Cubic Difference Tone, calculated by the formula 2F1-F2. Positive responses are normally detected 50 to 60dB below F1 intensity, when signal/noise ratio is higher than 6dB in at least 3 frequencies. However, it is important to bear in mind that both the distortion product intensity and noise levels are higher in children than adults. This method of otoacoustic emissions has some advantages over the others, since in addition to being less influenced by noise, it enables assessment of frequencies up to 8kHz, conveying much higher precision of frequency and location of cochlear damage, which may be essential for hearing aid fitting. The main disadvantage is high cost.
As to the practical aspects of otoacoustic emissions in general, they should always be performed in a silent environment. According to Kemp, 24-hour newborns are the best candidates to undergo otoacoustic emissions since they probably no longer have amniotic fluid in the external auditory canal, are accessible to the screening facilities, sleep for long periods of time, and are relatively free from middle ear effusion.
The advantages of otoacoustic emissions compared to BERA are: it requires less preparation of the child, it is quicker and easy to interpret. In addition, costs are accessible, they test all auditory peripheral structures, and are highly susceptible to auditory thresholds above 30dB. The disadvantages of otoacoustic emissions are no susceptibility to noise, the fact that they are influenced by dysfunction of other structures than the cochlea, such as middle or external ear, and the likelihood of not detecting retrocochlear affection, such as hyperbillirubinemia hearing loss or acoustic neuroma. Another problem of otoacoustic emissions is low specificity, which causes referral rates for post-screening auditory assessment of 10 to 20%. Nevertheless, the situation may be minimized by repeating the test, which reduces referral to 3 - 5%.
Models of hearing screening
The ideal screening method for childhood hearing has been topic for constant discussions. There are multiple options using different instruments and no consensus has yet been reached.
The model for Universal Screening advocated by NIH is the preferred one (Chart 6). This type of assessment combines high sensitivity and technical facility of otoacoustic emissions with the specificity of BERA. It allows effective identification of 100% of the severe hearing impaired children without causing excessive number of false-positive, which would lead to an excessive number of follow-up visits.
Conversely, the Screening of Risk Children is also a source of controversy considering the methods to use. Thus, whereas some defend OAE followed by BERA, others advocate BERA as the first line test.
Regardless of the situation, close attention to new techniques and technology is constantly required, because new methods and combinations, such as automated distortion product and BERA, shall soon take the center stage.
assessment of the hearing impaired child
The objectives of the assessment of potentially hearing-impaired children are the confirmation of severe hearing loss, identification of etiology and screening of other affections. Therefore, a careful clinical history is required, which is probably the most productive portion of the assessment, and the appropriate objective tests, comprising not only ENT examination but also general information about the child. Audiological assessment is performed with BERA and otoacoustic emissions, together with Behavioral Tests and Immittance Measures. Computed tomography is especially indicated in cases of meningitis, sudden or progressive deafness, or in children who have cochlear implants, in which we have to confirm the permeability of the cochlear duct. The assessment should also include general lab test and sometimes the patients have to be referred to other specialties, especially Genetics, Ophthalmology and Pediatrics.
Finally, during assessment, we should always provide parental support. The announcement of a case of deafness, especially if the parents did not suspect of anything, is always a great chock manifested by denial, fear, guilt and depression. Thus, the team should be sensitive to family repercussions and provide information about the disease and its consequences, encouraging the dialogue with the child and supporting the family in their choice of therapeutic methods and educational lines. As stated by Webster "it is impossible to hurt anyone by simply listening to them and providing the information they need. In such kind of counseling, the clinician is a spectator, putting aside his or her personal beliefs, and trying to accept the parents as they are and will be.".
Conclusion
Childhood deafness is an extremely important fact for Otorhinolaryngologists, Pediatricians and Audiologists, not only because of its consequences, but also owing to the multiple problems considering diagnostic and therapeutic issues. There are a number of therapeutic options currently available and we should be ready to provide the best we know to these children. Since the evolution of knowledge is becoming faster and faster, it justifies the need for constant updating,
REFERENCE
1. BEN, F.S.; PARADISE, J.L.: Universal screening for infant hearing impairment. Not simple, not risk-free, not necessarily beneficial and not presently justified. Pediatrics. 93: 330-4. 1993.
2. EREMBERG, A; LEMON, J.; SIA, C.; TRUNKEL, D.; ZIRING, P.: American Academy of Pediatrics - Task force on newborn and infant hearing. Newborn and infant hearing loss: detection and intervention. Pediatrics. 103:527-30. 1999.
3. FERNANDEZ, A.M.; IBARGUEN, A.M.; MONTOYA, F.S.; REY, A.S.: Parametros de la normalidad de las otoemissiones acústicas transitórias. Acta Otorrinolaringológica Espanola. 46: 339-44. 1995.
4. FRANÇOIS, M.: Classification et traitment des surditès de l'enfant. Encyclopedie Medico-chirurgicale ORL. 20190 C20 4061 E20. 1991.
5. FRANÇOIS, M.: Strategie diagnostique chez un enfant sourd. Encyclopedie Medico-chirurgicale ORL. 20190 C10 4061 E10. 1991.
6. GHANE, P.: Suppliance instrumentale de la surdité: les aides auditives. Revue Officielle de la Societé Française d'ORL. 54: 29-40. 1999.
7. GRANDOI, F.: European consensus development conference on neo-natal hearing screening - 1998. Arch. Otolaryngology Head and Neck Surgery. 125: 118. 1999.
8. HORN, K.L.; McDANIEL, S.L.: Early identification and intervention of hearing-impaired infants. The Otolaryngologic Clinics of North America. 32: 969-1141. 1999.
9. ISAACSON, G.: Pediatric Otology. The Otolaryngologic Clinics of North America. 27: 431-633. 1994.
10. JOINT COMMITTEE ON INFANT HEARING 1994: Position Statement. AAO-HNS Bulletin. 13. 1994.
11. KANE, T.J.; SCHAEFER, L.; PERKINS, J.A.: Potential pitfalls of initiating a newborn hearing screening program. Archives of Otolaryngology - Head and Neck Surgery. 125: 28-32. 1999.
12. KIMBERLEY, B.P.: Applications of distortion-product emissions to an otological practice. The Laryngoscope. 109: 1908-18. 1999.
13. LAFRANIERE, D. et al: Otoacoustic emissions in full-term newborns at risk for hearing loss. The Laryngoscope. 103: 1334-41. 1993.
14. LEVI, H. et al: Transient-evoked otoacoustic emissions in newborns in the first 48 hours after birth. Audiology. 36: 181-86. 1997.
15. LEWER, R.M.; BERTULEIT, H.; WITTKUGEL, O.; FREITAG, H.J.: Indications for imaging the inner ear with CISS-MRI. Laryngorhinootologie. 78: 417-20. 1999.
16. MEYER, C. et al: Neonatal screening for hearing disorders in infants at risk: incidence, risk factors and follow-up. Pediatrics. 104:900-4. 1999.
17. NATIONAL INSTITUTE OF HEALTH: Consensus development conference statement: early identification of hearing impairment in infants and young children. Int. Journal Pediatric Otorhinolaryngology. 27: 215-227. 1993.
18. REE, C.; PARK, H.; JANG, Y.: Audiologic evaluation of neonates with severe hyperbillirubinemia using transiently evoked otoacoustic emissions and auditory brainstem responses. The Laryngoscope. 109: 2005-8. 1999.
19. SERRANO, M.M.; LATORRE, A.A.; SAINZ-TRAPAGA, C.S.: Detection precoz de la sordera en la infancia. J. Esp. Pediatria. 46: 534-7. 1997.
20. VAN STRAATEN, H.L.: Automated auditory brainstem response in neonatal hearing screening. Acta Paediatr. 88: 76-79. 1999.
[1] Complementary Internship in Otorhinolaryngology.
[2] Hospital Assistant in Otorhinolaryngology
[3] Director of the Service of Otorhinolaryngology
Affiliation: Service of Otorhinolaryngology, Centro Hospitalar de Vila Nova de Gaia
Address correspondence to: Centro Hospitalar de Vila Nova de Gaia - Serviço de O.R.L.
Rua Conceição Fernandes 4434-502 Vila Nova de Gaia - Porto - Portugal -
Tel: 351 22 7865100 - Ext. 1433
E-mail: pedro.j.oliveira@mail.pt