Year: 2002 Vol. 68 Ed. 3 - (16º)
Artigo Original
Pages: 400 to 403
Quality of life and prognosis of squamous cell carcinoma of the head and neck
Author(s):
Ali Amar(1),
Abrão Rapoport(1),
Sérgio A. Franzi(1),
Clarice Bisordi(2),
Carlos N. Lehn(1)
Keywords: head and neck neoplasms, squamous cell carcinoma, quality of life
Abstract:
Aim: a quality life questionnaire (QLQ) assessment and its relationship with prognostic of patients with head and neck cancer. Study design: prospective clinical. Material and method: a study of 31 patients with squamous cell carcinoma of upper aerodigestive tract submitted to therapy in Head and Neck Surgery and Otorhinolaryngology Department of Heliópolis Hospital, Hosphel between August,1999 and November, 2000. For evaluation of quality of life it was used the questionnaire QLQ-C30 and the module QLQ-H&N35, both supplied by EORTC, accomplished previously to the treatment. The differences of scores among patients with controlled disease and those with recurrence in the first 12 months after treatment was evaluated. Results: a significant difference was observed in scores between assimptomatic patients and those with recurrence, when related to the fadiga, problems of eating in public and global quality of life (p £ 0,05). The scores in this group were similar to those observed in other populations. Conclusion: the pre-treatment quality of life can be a prognostic indicator for patients with head and neck cancer.
![]()
INTRODUCTION
Evaluation of quality of life has gained attention in head and neck tumor management, since it normally causes esthetical and functional compromise. Based on the use of organ and structure preservation protocols, quality of life has attracted more interest and it became an argument to justify the less mutilating therapeutic modalities when oncologic results are equivalent.
Many instruments were created to quantify quality of life. Even though it has become a popular term, the concept of quality of life reflects emotional, cultural and economic aspects far more complex than the questionnaires developed to quantify it. In Brazil, a significant number of patients come from poor educational background and low income levels. The effects of the disease in the routine of these patients and the use of instruments to evaluate quality of life have not been properly defined for this population yet. The need to quickly treat the patient with cancer bases therapeutic management almost only on staging of the disease, according to predefined schemes, which do not always take into account the benefits of the procedure from the patients' reported criteria (which is an extremely important bioethical principle). Patients react differently towards the treatment sequelae as they also value their symptoms differently. There is no correlation between the quality of life evaluated items and disease staging, but quality of life is related to prognosis1.
The present study intended to asses the quality of life measurement instrument [(QLQ-C30 = Quality of Life Questionnaire Core (30 questions) and QLQ-H&N35 = Quality of Life Questionnaire - Head and Neck Cancer Module (35 questions)] and their potential prognostic value in head and neck squamous cell carcinoma.
MATERIAL AND METHOD
We prospectively studied 31 patients with head and neck squamous cell carcinoma seen at the Department of Head and Neck Surgery and Otorhinolaryngology at Hospital Heliópolis, Hosphel, São Paulo, between August 1999 and November 2000.
As to primary tumor site, 12 patients had mouth tumor, 6 had laryngeal tumor, 8 oropharynx, 4 hypopharynx, and 1 paranasal sinuses tumor. As to staging, 2 had stage I disease, 3 had stage II diseases, 9 had stage III and 17 had stage IV. Surgical treatment was employed in 26 patients, whereas 5 of them were submitted to radiotherapy. As to level of education, 2 patients were illiterate, 23 had incomplete elementary education, 2 had complete elementary education, 1 had incomplete high school education, and 3 had complete high school education. Twenty-eight patients were male and 3 were female.
As to quality of life evaluation, we used the questionnaire QLQ-C30 version 3, with the inclusion of the module QLQ-H&N35, both of them developed by EORTC - European Organization for Research and Treatment of Cancer. Questionnaires were covered by the same examiner with all patients and before treatment.
QLQ-C30 has 30 questions grouped in 5 functional scales, 9 scales related to symptoms and one global scale. QLQ-H&N35 has 35 questions, out of which 30 are grouped in 13 scales and 5 simple-answer questions. Answers were converted into a linear grading scale, ranging from 0 to 100, as advocated by EORTC. Results were expressed in means, with confidence interval. High score in questions related to symptoms reflected the more marked presence of them, whereas high score in questions related to function reflected better physical capacity of patients.
We assessed scores concerning the different primary sites, disease staging and recurrence (or persistence) in the 12 post-treatment months. The differences between groups were analyzed by Mann-Whitney test, considering alpha error equal or below 5%.
RESULTS
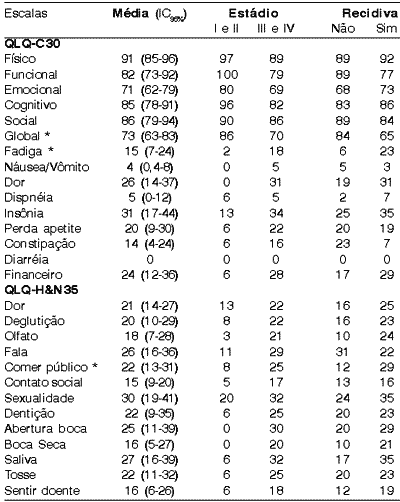
Table 1 presents the score attained for each scale of questionnaire QLQ-C30 and QLQ-H&N35 concerning disease staging and control.
We observed statistically significant differences in the scales related to fatigue (p=0.03), global quality of life (p=0.05) and difficulty to eat in public (p=0.03) between patients with controlled disease and those who had a recurrence within the first 12 months after treatment. Considering only these three scales, the recurrence (or persistence) rate of the disease between the patients with unfavorable score in at least two scales was 90% (10/11), whereas 65% (13/20) of those who had favorable scores in at least two scales had the disease controlled by the end of the 12 months.Table 1. Mean score of QLQ-C30 and QLQ-H&N35 questionnaires.
* significant variables in controlled patients or in those with recurrence 12 months after treatment.
DISCUSSION
Although it is assumed that diseases in more advanced stages have more symptoms, cause more dysfunction and, consequently, are related to worse quality of life, it may apply to one individual but does not reflect the reality of a heterogeneous group of patients. Even if the relation between staging and quality of life can be defined in larger samples, we should not disregard wide individual variation, because patients who have the disease in similar stages may value differently their symptoms and limitations. Therefore, in order to assess the effects of different therapeutic modalities on patients' quality of life, we should also evaluate quality of life before treatment and individual variation, because scoring scale is a weakness of questionnaires. The moment the evaluation is made also influences results, because there is a decrease in quality of life immediately after treatment, with gradual restoration of baseline values (pre-treatment) after an interval of about 6 months. The control of the disease may cause improvement of emotional functions, although there may also be decrease in physical functions2. Characteristics of the different therapeutic modalities concerning sequelae and rehabilitation time should also be considered.
In addition to the scale related to fatigue, the ones related to difficulty to eat in public and global quality of life also showed correlation with recurrence of the disease in the first 12 post-treatment months. As a result of staging limitation and histopathology to define individual prognosis considering the therapeutic modalities available, the aspects concerning quality of life should be further investigated. The present study sample is heterogeneous and too small to be submitted to multivariate analysis, because the interpretation of these findings require caution, since the motivation of treatment and the presence of comorbidity should not be forgotten in the therapeutic planning.
Depression is a frequent finding in patients with cancer, although it is not always appropriately addressed by the oncologist3. In addition to interfering in quality of life, depression can affect the immune system, favoring the recurrence of the disease. The relation between psychosocial factors and cancer progression is an area of recent interest and considering the complexity of the subject, there are still no conclusive studies4, 5.
In Brazil, most patients come to the specialist with advanced staged diseases (stages III and IV) and they are normally submitted to combined treatment. The duration of treatment, considering pretreatment tests, length of hospital stay (surgery) and radiotherapy treatment is approximately 2 to 3 months. Treatment morbidity and its definite sequelae significantly compromise quality of life of the patients during the period. Rehabilitation, especially concerning speech and swallowing, can also take long, which explains why it takes long for the patients to recover their baseline quality of life. Early recurrence reveals the physician's limitation to indicate the treatments with high morbidity, even if they are the only (and limited) opportunity of cure. Therefore, quality of life should also be considered for patients that do not have control of the disease, assessing the difference between preservation of structures (to preserve organs imply to preserve function) and "hygienic" surgery.
The questionnaire can be answered directly by the patients, but the low educational level of most patients required the presence of an interviewer, compromising the uniform application of questionnaires because it required adaptations for comprehension purposes, considering the low educational level of the group. EORTC questionnaire was formulated to allow comparison between different populations. The similarity between the scores of the present study and those reported by studies conducted in Europe suggests that the questionnaire has attained its goal2, 6 and, therefore, should be extended to more patients.
CONCLUSION
Quality of life previous to treatment may be a relevant prognostic indicator for patients with head and neck cancer.
REFERENCES
1. Hammerlid E, Wirblad B, Sandin C. Malnutrition and food intake in relation to quality of life in head and neck cancer patients. Head Neck 1998;20:540-8.
2. Hammerlid E, Silander E, Hörnestam RN. Health-related quality of life three years after diagnosis of head and neck cancer - A longitudinal study. Head Neck 2001;23:113-25.
3. Bukberg J, Penman D, Holland JC. Depression in hospitalized cancer patients. Psychosom Med 1984;46:199-212.
4. De Graeff A, De Leeuw JRJ, Ros WJG. Pretreatment factors predicting quality of life after treatment for head and neck cancer. Head Neck 2000;22:398-407.
5. Garssen B, Goodkin K. On the role of immunological factors as mediators between psychosocial factors and cancer progression. Psychiatry Res 1999;85:51-61.
6. Aaronson NK, Ahmedzai S, Bergman B. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality of life instrument for use in international clinical trials. J Natl Cancer Inst 1993;85:365-76.
[1] Surgeon, Department of Head and Neck Surgery and Otorhinolaryngology, Hospital Heliópolis, Hosphel, São Paulo.
[2] Psychologist, Department of Head and Neck Surgery and Otorhinolaryngology, Hospital Heliópolis, Hosphel, São Paulo.
Study conducted in the Department of Head and Neck Surgery and Otorhinolaryngology, Hospital Heliópolis, Hosphel, São Paulo.
Address correspondence to: Abrão Rapoport - Rua Iramaia, 136 Jd. Europa - 01450-020 - São Paulo SP - Tel. (55 11) 273.8224 - E-mail: cpgcp.hosphel@attglobal.net