Year: 2012 Vol. 78 Ed. 1 - (16º)
Artigo Original
Pages: 103 to 108
Comparison of the area of the pharynx during wakefulness and induced sleep in patients with Obstructive Sleep Apnea (OSA)
Author(s): Ana Célia Faria1; Luis Vicente Garcia2; Antonio Carlos dos Santos3; Paula Rejane Beserra Diniz4; Helcio Tadeu Ribeiro5; Francisco Veríssimo de Mello-Filho6
Keywords: magnetic resonance imaging, obstructive, pharynx, sleep apnea.
Abstract:
The study of obstructive sleep apnea (OSA) has received growing attention over the past years since various aspects have not been sufficiently established.
AIM: To evaluate, with the use of magnetic resonance imaging (MRI), changes in the area of the pharynx during wakefulness and induced sleep in patients with OSA.
MATERIALS AND METHODS: A prospective study of thirty-two patients with a polysomnographic diagnosis of OSA. All patients were submitted to MR imaging in order to obtain high-definition anatomical sagittal sequences during wakefulness and during sleep induced with Propofol. An area was defined on the sagittal plane in the midline of the pharynx. This region was called pharyngeal midplane (PMP) area.
RESULTS: A significant difference in PMP area (mm2) was observed between wakefulness and induced sleep in each patient (p < 0.000001).
CONCLUSION: The patients with OSA suffer a significant reduction of 75,5 % in the area of the pharynx during induced sleep compared to wakefulness.
![]()
INTRODUCTION
The obstructive sleep apnea syndrome (OSAS) has been studied in greater depth to investigate several relevant aspects of its physiology pathology and therapy that are not sufficiently clear. A clear understanding of upper airway structures and function in OSAS patients is needed to further understand the pathogenesis of this disorder and to select the most appropriate therapy.
With this in mind, the site of obstruction in upper airways has been studied using several approaches, such as the physical examination, nasopharyngolaryngoscopy, and image methods - cephalometrics, computed tomography (CT), and magnetic resonance (MR) imaging1. A limitation of methods that evaluate OSAS is that they are done with patients awake, often in a sitting or standing position, and thus do not faithfully reproduce the upper airway morphology during sleep. It is, therefore, not always possible when patients are awake to identify even a simple narrowing of upper airways as a cause of the events involved in airway occlusion during sleep.
MR imaging has been used in OSAS patients to locate oropharyngeal obstructions and abnormalities2, to study upper airways and adjacent soft tissues3-8, to assess fat deposits in airways9,10, to find anatomical factors in airways at risk for OSAS11, and to study possible anatomical differences in the airways of males and females12. A functional assessment of patients in decubitus and sleeping is needed to locate any obstruction, as the morphology of the airways varies when patients are awake or sleeping13.
Polysomnography is done while patients are asleep. It is able to detect disease severity, and whether it is of central or obstructive origin; this method, however, is unable to detect an obstruction site in upper airways. Thus, morphological studies of upper airways are routinely carried out with patients awake in an attempt to locate the obstruction site. This difference may be the cause of difficulties to identify anatomical obstruction sites, and to indicate the most appropriate procedure for the treatment of OSAS.
There have been few published studies using MR imaging to assess OSAS patients in the sleeping state14-18. The emphasis in these studies has been either to compare the morphology of OSAS patients with that of normal controls or to establish a relationship between disease severity and obstruction sites.
We found no studies on differences in pharyngeal area (mm2) in OSAS patients measured in wakefulness and sleep.
Objective
The purpose of this study was to assess changes in the pharyngeal area of OSAS patients in wakefulness and induced sleep by using MRI.
MATERIALS AND METHODS
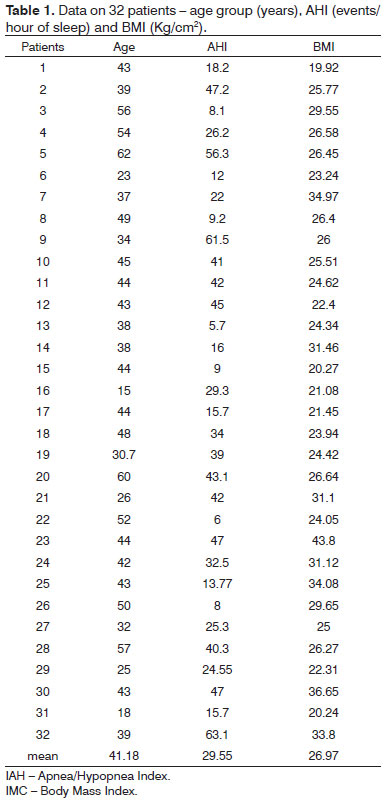
The sample comprised 32 patients aged from 18 to 62 years, diagnosed with OSAS. The inclusion criterion was OSAS demonstrated by polysomnography as defined by an apnea/hypopnea index (AHI) over five events per hour. Exclusion criteria were use of medication and neuromuscular diseases. The clinical history and physical examination based on a standard protocol at our unit were part of the procedure for all patients in the sample. The institutional review board approved this study on human beings (protocol number 6245/2006).
Polysomnography
Polysomnography was done in the Sleep Laboratory of the Clinical Neurophysiology Section of the hospital; it consisted of an entire night recording. Patients were admitted from 7 to 10 p.m. and discharged at 7 a.m. on the next morning.
At the sleep laboratory, patients answered a standard questionnaire before and after polysomnograph recordings. A Digital BioLogic Polysomnograph - Poliwin 2000 software was used, and the recording time was at least 5 hours. The study variables were grouped into an internationally proposed standard format for polysomnography.
Magnetic Resonance Imaging
All patients were referred to the Image Unit of the hospital for MR imaging, which was done on a Magneton Vision 1.5 Tesla superconducting device (Siemens, Erlangen, Germany) with a 25 milliTesla gradient magnetic field, and an emit and receive radiofrequency head coil with a circular polarized component.
The image acquisition protocol included anatomical high-definition sagittal T1-weighted images followed by a dynamic study with T1-weighted fast ecogradient sequences with patients awake.
Next, sleep was induced by endovenous Propofol (3 to 5 mg per Kg) by an anesthesiologist, after which images were again acquired as soon as patients were asleep.
DICOM images were transferred to an auxiliary workstation.
Analysis of images
Measurements were taken using the Display software (McGill University, Montreal, Canada).
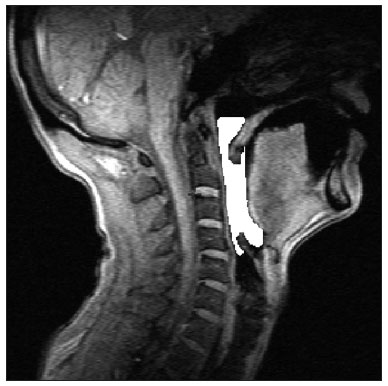
The area that was assessed was only the air space; hard or soft tissues were not included. This area was defined on the sagittal plane of the pharyngeal midplane, superiorly by a line passing along the hard palate to the posterior pharyngeal wall, inferiorly by the back of the vallecula, posteriorly as the posterior pharyngeal wall, and anteriorly as the base of tongue and the soft palate (Figure 1). This region was named the pharyngeal midplane (PMP) area. The PMP area was measured pixel by pixel in sagittal images using the abovementioned software, which yields simultaneous views of three orthogonal planes and makes it possible to accurately define the anatomy. It generates a binary file from which the area may be calculated.
Figure 1. Image showing the PMP area defined superiorly by a line passing along the hard palate to the posterior pharyngeal wall, inferiorly by the back of the vallecula, posteriorly as the posterior pharyngeal wall, and anteriorly as the base of tongue and the soft palate.
Analysis of the PMP area of patients awake and under induced sleep
The first step was an analysis on the sagittal plane to compare the area of interest in the same patient while awake and in induced sleep. Two sequences of the same spatial location were used, one section with the patient awake and one with the patient in induced sleep. The PMP was drawn by thresholding to measure the maximum size (mm2). Only the maximum PMP measure was used, as the shortest measure, generally seen when patients were in induced sleep, was collapse of the area. This procedure avoided including other areas that were not of interest for this study, and reduced interference of the partial volume as much as possible. The software calculated the areas automatically and inserted the numbers into spreadsheets for statistical comparisons.
RESULTS
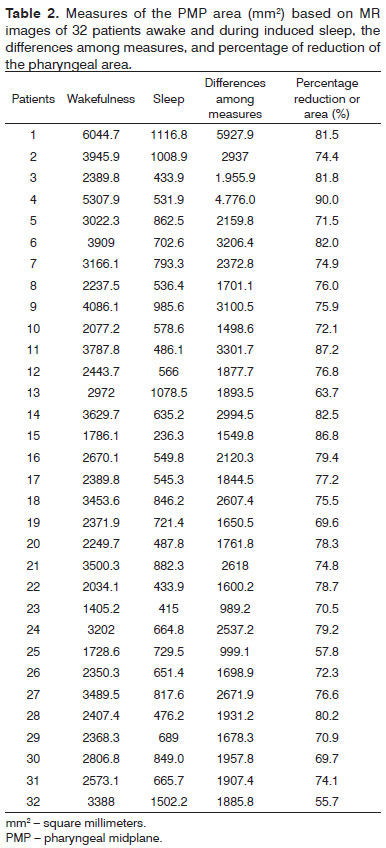
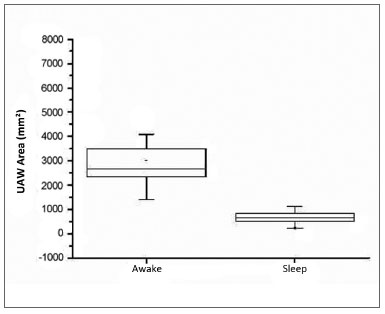
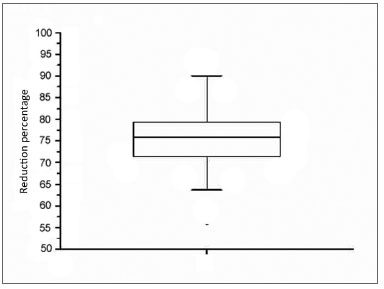
There were 23 male and nine female patients in the sample of 32 OSAS subjects. The mean AHI was 29.55 events per hour of sleep; the mean BMI was 26.97 (Table 1). Table 2 shows the PMP measures (mm2) and wakefulness and induced sleep for each patient, the differences among these measurements, and the percentage reduction of the pharyngeal area during induced sleep. A comparison of values gathered from patients awake and in induced sleep yielded a statistically significant difference (p< 0.000001). The upper airway values in wakefulness and induced sleep are presented graphically based on its distribution in mm2 (Chart 1). The mean reduction of the pharyngeal area during induced sleep was 75.55% (SD 7.44) (Chart 2).
Chart 1. Distribution of area measurements (mm2) of upper airways in wakefulness and induced sleep.
Chart 2. Graphic representation of the percentage reduction of the upper airway area during induced sleep.
DISCUSSION
Because surgery for the treatment of OSAS results in permanent anatomical changes and do not require patient compliance after the procedure is undertaken, studies of the preoperative anatomy of upper airways may significantly help select the ideal technique19. Therefore, this study presents a standard objective approach for preoperative evaluation in which the reduction of the upper airway area may be defined numerically while the patient is asleep.
A few studies have shown that OSAS patients generally present a reduced airway space, which may lead to occlusion during sleep15. These studies, however, have not objectively measure this reduction.
We found in this study that although the upper airways are wide enough for air to pass when patients are awake, they undergo a major reduction in the area (75%) during sleep, resulting in obstruction (Table 2 and Chart 2). This is an important finding, as we found no similar result in the medical literature.
Suto et al.14 (1993) published a study of 15 OSAS patients in which the upper airways were assessed subjectively by MR with patients awake and in induced sleep. Their results indicated significant anatomical changes in the upper airways of 87% of their sample when evaluated during induced sleep. Our results, however, show in a plain and measurable way that the PMP area was significantly reduced in 100% of the study sample during sleep.
Such a significant change in the upper airways appears to determine the severity of OSAS, as Suto & Inoue15 have reported; these authors concluded that multiple obstruction sites in the pharynx are associated with severe apnea.
The size of the airway in the pharynx is determined by an interaction between neural regulation of dilatory muscle activity and structure. Pharyngeal collapse during sleep in OSAS patients could therefore be cause by abnormalities of these factors20.
Anatomical factors, such as craniofacial abnormalities (retrognathism, micrognathism, maxillary atresia, overbite), an abnormal soft palate, macroglossia, hypertrophic tonsils, and nasal block, may contribute to the onset of apnea21. There are, however, OSAS patients that have no obvious anatomical changes. Our results showed that the method we described may identify and measure area reductions in these cases.
Abbey et al.22 (1989) suggested that OSAS patients do not always present a narrow pharynx while awake, but that they may have variable sites of narrowing in the upper airways that collapse during sleep. Thus, especially in patients with no obvious anatomical changes, function (coordination of pharyngeal dilatory muscles) becomes important. A morphological assessment of upper airways is essential during wakefulness and sleep as the pharyngeal dilatory muscle activity differs in these two states.
Our results demonstrate a significant reduction (p< 0.000001) of the air space during induced sleep - with collapse sites within the PMP area - in the entire sample. These anatomical changes do not appear and cannot be diagnosed when patients are tested awake. Thus, MR imaging in wakefulness and induced sleep was able to accurately identify and measure upper airway changes.
CONCLUSION
The mean pharyngeal area of OSAS patients in our sample was significantly reduced - by 75.55% - during induced sleep compared to wakefulness.
REFERENCES
1. Stuck BA, Maurer JT. Airway evaluation in obstructive sleep apnea. Sleep Med Rev. 2008;12(6):411-36.
2. Suto Y, Matsuda E, Inoue Y, Suzuki T, Ohta Y. Sleep apnea syndrome: comparison of MR imaging of the oropharynx with physiologic indexes. Radiology. 1996;201(2):393-8.
3. Schwab RJ, Gupta KB, Gefter WB, Metzner LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;152(5 Pt 1):1673-89.
4. Schwab RJ. Properties of tissues surrounding the upper airway. Sleep. 1996;19(10 Suppl):S170-4.
5. Ciscar MA, Juan G, Martínez V, Ramón M, Lloret T, Mínguez J, et al. Magnetic resonance imaging of the pharynx in OSA patients and healthy subjects. Eur Respir J. 2001;17(1):79-86.
6. Mariën S, Schmelzer B. Velopharyngeal anatomy in snorers and patients with obstructive sleep apnea. Acta Otorhinolaryngological Belg. 2002;56(2):93-9.
7. Stuck BA, Köpke J, Maurer JT, Verse T, Kuciak G, Düber C, et al. Evaluating the upper airway with standardized magnetic resonance imaging. Laryngoscope. 2002;112(3):552-8.
8. Welch KC, Foster GD, Ritter CT, Wadden TA, Arens R, Maislin G, et al. A novel volumetric magnetic resonance imaging paradigm to study upper airway anatomy. Sleep. 2002;25(5):532-42.
9. Shelton KE, Woodson H, Gay SB, Suratt PM. Adipose tissue deposition in sleep apnea. Sleep. 1993;16(8 Suppl):S103-5.
10. Schäfer H, Pauleit D, Sudhop T, Gouni-Berthold I, Ewig S, Berthold HK. Body fat distribution, serum leptin, and cardiovascular risk factors in men with obstructive sleep apnea. Chest. 2002;122(3):829-39.
11. Schwab RJ, Pasirstein M, Pierson R, Mackley A, Hachadoorian R, Arens R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168(5):522-30.
12. Daniel MM, Lorenzi MC, da Costa Leite C, Lorenzi-Filho G. Pharyngeal dimensions in healthy men and women. Clinics. 2007;62(1):5-10.
13. Rama AN, Tekwani SH, Kushida CA. Sites of obstruction in obstructive sleep apnea. Chest. 2002;122(4):1139-47.
14. Suto Y, Matsuo T, Kato T, Hori I, Inoue Y, Ogawa S, et al. Evaluation of the pharyngeal airway in patients with sleep apnea: value of ultrafast MR imaging. AJR Am J Roentgenol. 1993;160(2):311-4.
15. Suto Y, Inoue Y. Sleep apnea syndrome. Examination of pharyngeal obstruction with high-speed MR and polysomnography. Acta Radiol. 1996;37(3 Pt 1):315-20.
16. Yokoyama M, Yamanaka N, Ishii H, Tamaki K, Yoshikawa A, Morita R. Evaluation of the pharyngeal airway in obstructive sleep apnea: study by ultrafast MR imaging. Acta Otolaryngol Suppl. 1996;523:242-4.
17. Donnelly LF, Surdulescu V, Chini BA, Casper KA, Poe SA, Amin RS. Upper airway motion depicted at cine MR imaging performed during sleep: comparison between young patients with and those without obstructive sleep apnea. Radiology. 2003;227(1):239-45.
18. Chuang LP, Chen NH, Li HY, Lin SW, Chou YT, Wang CJ, et al. Dynamic upper airway changes during sleep in patients with obstructive sleep apnea syndrome. Acta Otolaryngol. 2009;129(12):1474-9.
19. Huang Y, White DP, Malhotra A. Use of computational modeling to predict responses to upper airway surgery in obstructive sleep apnea. Laryngoscope. 2007;117(4):648-53.
20. Okubo M, Suzuki M, Horiuchi A, Okabe S, Ikeda K, Higano S, et al. Morphologic analyses of mandible and upper airway soft tissue by MRI of patients with obstructive sleep apnea hypopnea syndrome. Sleep. 2006;29(7):909-15.
21. Fleisher KE, Krieger AC. Current trends in treatment of obstructive sleep apnea. J Oral Maxillofac Surg. 2007;65(10):2056-68.
22. Abbey NC, Block AJ, Green D, Mancuso A, Hellard DW. Measurement of pharyngeal volume by digitized magnetic resonance imaging. Effect of nasal continuous positive airway pressure. Am Rev Respir Dis. 1989;140(3):717-23.
1. Doctoral degree in medical science, Ribeirão Preto Medical School, São Paulo University. Oral and maxillofacial surgeon, Integrated Center for the Study of Facial Deformities (Centro Integrado de Estudo das Deformidades da Face or CIEDEF) Clinic Hospital, Ribeirão Preto Medical School, São Paulo University.
2. Adjunct professor, Biomechanics, Medicine and Rehabilitation of the Aparelho Locomotor Department, Ribeirão Preto Medical School, São Paulo University.
3. Associate professor, Internal Medicine Department, Clinic Hospital, Ribeirão Preto Medical School, São Paulo University.
4. Master's degree in neuroscience, bachelor degree in biomedical informatics, doctoral student, Neuroscience Department, Ribeirão Preto Medical School, São Paulo University.
5. Specialist in orthodontics, doctoral student, Ophthalmology, Otorhinolaryngology and Head & Neck Surgery Department, Ribeirão Preto Medical School, São Paulo University.
6. Associate professor and head of the Integrated Center for the Study of Facial Deformities, Ophthalmology, Otorhinolaryngology and Head & Neck Surgery Department, Ribeirão Preto Medical School, São Paulo University.
This study was carried out at the Integrated Center for the Study of Facial Deformities (Centro Integrado de Estudo das Deformidades da Face or CIEDEF) Clinic Hospital, Ribeirão Preto Medical School, São Paulo University (USP).
Send correspondence to:
Ana Célia Faria
Departamento de Oftalmologia, Otorrinolaringologia e Cirurgia de Cabeça e Pescoço da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo
Av. Bandeirantes, 3900
Ribeirão Preto - SP, Brazil. Zip Code: 14049-900
Phone: (16) 3602-2523.
Paper submitted to the BJORL-SGP (Publishing Management System - Brazilian Journal of Otorhinolaryngology) on April 28, 2011.
Accepted on October 2, 2011. Code: 7732.