Year: 2012 Vol. 78 Ed. 1 - (12º)
Artigo Original
Pages: 75 to 80
Comparison between endoscopic and open surgery in 37 patients with nasopharyngeal angiofibroma
Author(s): José Alberto Alves Oliveira1; Marylane Galvão Tavares2; Carolina Veras Aguiar3; Jorge Ferreira de Azevedo4; João Renato F. Sousa5; Paulo César de Almeida6; Erika Ferreira Gomes7
Keywords: angiofibroma, neoplasm staging, video-assisted surgery.
Abstract:
Juvenile nasopharyngeal angiofibroma is a rare benign vascular tumor of the nasopharynx. Although the treatment of choice is surgery, there is no consensus on what is the best approach.
AIM: To compare surgical time and intraoperative transfusion requirements in patients undergoing endoscopic surgery versus open / combined and relate the need for transfusion during surgery with the time between embolization and surgery.
MATERIAL AND METHODS: Study descriptive, analytical, retrospective study with a quantitative approach developed in the Otorhinolaryngology department of a teaching hospital. Analyzed 37 patients with angiofibroma undergoing surgical treatment. Data obtained from medical records. Analyzed with tests of the Fisher-Freeman-Halton and Games-Howell. Was considered significant if p <0.05.
STUDY DESIGN: Historical cohort study with cross-sectional.
RESULTS: The endoscopic approach had a shorter operative time (p <0.0001). There is less need for transfusion during surgery when the embolization was performed on the fourth day.
CONCLUSION: This suggests that the period ahead would be ideal to perform the process of embolization and endoscopic surgery by demanding less time would be associated with a lower morbidity. This study, however, failed to show which group of patients according to tumor stage would benefit from specific technical.
![]()
INTRODUCTION
The juvenile nasopharyngeal angiofibroma is a rare, highly vascular benign tumor of the nasopharynx. It comprises about 0.5% of all head and neck neoplasms. The incidence is 1:150.000 and it affects males in the 14 to 25 years age group1.
Accumulation in the nucleus of β-catenin, a co-activator of androgenic receptors, may explain why this tumor selects the male sex. Normal serum levels of hormones in patients with juvenile nasopharyngeal angiofibroma corroborate this explanation2.
The typical presentation of juvenile nasopharyngeal angiofibroma consists of chronic nasal block, epistaxis, rhinorrhea, and pain. Because of its invasive growth, it may lead to facial deformity, proptosis, abnormal visual acuity if the orbit is involved, and paralysis of cranial nerves if there is intracranial involvement2,3.
A correct diagnosis is made based on the clinical history, the otorhinolaryngologic physical examination, and imaging; these procedures may avoid the need for biopsy, with its risk of hemorrhage. The radiologic exam of choice is contrasted computed tomography of the nose and paranasal sinuses; a tumor in the nasopharynx with widening of the sphenopalatine foramen and marked contrast uptake may be seen. Magnetic resonance imaging is used if there is cranial and orbitary invasion. Digital subtraction selective angiography may be used for the diagnosis and treatment by identifying the blood vessels that feed the tumor and offering the opportunity to embolize these vessels preoperatively3,4.
Surgery is the treatment of choice for juvenile nasopharyngeal angiofibroma; there are also published descriptions of radiotherapy, hormone therapy, cryotherapy, electrocoagulation, and chemotherapy. Surgery for juvenile nasopharyngeal angiofibroma may be endoscopic, open, or combined5-7.
The choice of surgery is based on the extent of the tumor and the surgeon's experience. Staging is based on any of the Radkwoski, Chandler, Sessions, Andrews, or Fisch classifications; the latter is used more often3.
Endoscopic surgery is a possible treatment approach when tumors are in stages I and II (Fisch) because the morbidity is lower.6 It may also be used together with traditional surgery to reduce operative complications and tumor recurrences8.
The purpose of this study was to compare the duration of surgery in each approach (open, endoscopic, and combined surgery), and to assess the need for intraoperative blood transfusion compared to the time elapsed since embolization.
MATERIAL AND METHOD
A retrospective, descriptive, analytic, quantitative, cross-sectional cohort study was carried out at the otorhinolaryngology unit of a tertiary teaching hospital.
The files of patients operated from 2001 to 2010 were reviewed. The sample comprised 37 patients who underwent surgical removal of a nasopharyngeal angiofibroma. The inclusion criteria were a histologic diagnosis of nasal angiofibroma at our institution, and having undergone preoperative embolization with alcohol-polyvinyl 150-350 µm particles.
The study variables were the general characteristics of patients (age and symptoms), of surgical procedures (duration, in minutes; transfusion; embolization; and type of approach), and tumor staging. Surgeries comprised two groups: endoscopic, and open/combined surgery. The surgical approach across ten years took into account the surgeon's learning curve for endoscopic surgery and the gradual replacement of the open technique.
The Fisch classification was used for staging: stage I (tumor limited to the nasopharynx and nasal cavity with no bone destruction), stage II (tumor invading the pterygomaxillary fossa, the maxillary antrum, the ethmoid and sphenoid sinuses with bone destruction), stage III (tumor invading the infratemporal fossa, the orbit, and the parasellar area, but remaining lateral to the cavernous sinus), and stage IV (tumor with invasion of the cavernous sinus, the optic chiasm or the pituitary fossa)3,9.
An analysis of the time elapsed between embolization and surgery was made to identify the day that concentrated most patients (fourth day) and compare it with the remaining days.
The same surgeons carried out all surgical procedures (E.F.G).
The SPSS version 16.0 statistics software was applied for data processing. Simple and percentage frequencies, parametric measures, means and the standard deviation were the basis for data analysis. The Games-Howell test was applied to analyze the difference between mean pairs, as the variances were unequal. The Fisher-Freeman-Halton test was applied for association analyses. The significance level was p < 0.05.
RESULTS
Of 37 patients that underwent surgery for the nasopharyngeal angiofibroma, the mean age was 17 ± 7 years, ranging from 8 years to 44 years. The maxillary artery was the blood vessel that irrigated the tumor in all cases. The ascending pharyngeal and the ophthalmic arteries also contributed to irrigate the tumor in one case, and two direct branches of the internal carotid fed the tumor in two other cases.
The time elapsed between embolization and surgery ranged from 1 to 14 days (mean 5 ± 3 days). The symptoms at the time of diagnosis were: epistaxis (96.8%), nasal block (87.8%), facial bulging (22.5%), nasal discharge (19.4%), ocular symptoms (16.1%), facial pain/headache (12.9%), and hypoacusis (3.2%).
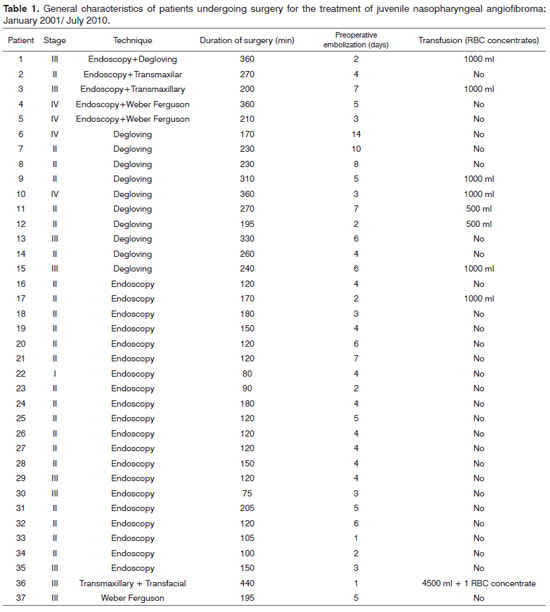
The distribution of patients according to tumor staging was as follows: stage I, one patient (3%); stage II, 23 patients (62%); stage III, nine patients (24%); and stage IV, four patients (11%) (Table 1).
Intraoperative transfusions were needed in nine patients. Of these, eight underwent open surgery and one underwent endoscopic surgery (Table 1).
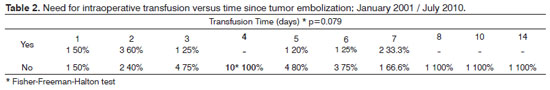
A comparison of patients in which embolization was done four days before surgery (most patients) with patients in which embolization was done on other days revealed that intraoperative transfusions were not required when embolization was done specifically on this day (p>0.05) (Table 2).
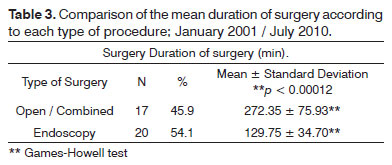
A comparison of the mean duration of surgery among the surgical procedures showed that endoscopy required less time than open/combined surgery (Table 3).
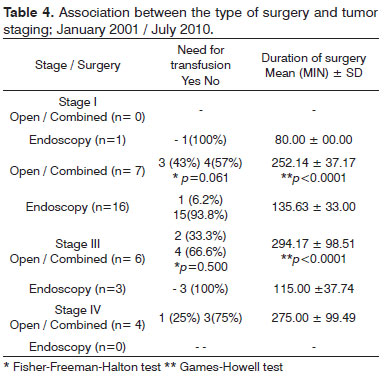
Most of the patients of all tumor stages (except for stage I) that required transfusions during surgery belonged to the open/combined surgery group. The mean duration of surgery was higher in this group compared to the group of patients that underwent endoscopy. There was, however, no significant relationship between tumor stage and the need for intraoperative transfusion (Table 4).
DISCUSSION
Prior embolization of the tumor makes it easier to identify anatomical landmarks during surgery without significant bleeding, thereby facilitating the procedure. Consequently, prior embolization also helps reduce surgical morbidity6.
Published papers have shown that the ideal time for embolization of tumors is one to three days before endoscopy8. However, our results showed that blood transfusion was less necessary when embolization was done four days before surgery compared to other number of days before; furthermore, surgeons found that the four day time period yielded a better cleavage plane and less perioperative bleeding.
Endoscopic surgery has many advantages compared to the traditional approaches. The main one is that a wider view of the tumor and anatomical landmarks from several angles becomes possible, which yields a better visualization of the interface between the tumor and adjacent soft tissues and bone. The result is a more accurate and complete dissection and better control of bleeding4,10. A further advantage of endoscopic surgery is that incisions on the skin and mucosa and facial osteotomies are unnecessary, thereby affecting less the growth of the midface of adolescents. Incisions and closure is not needed, which reduces the duration of surgery and postoperative complications, such as dysesthesia, pain, and trismus, which occur in some of the external approaches4.
Yiotakis et al. 9 studied the postoperative complications of 20 patients in which a nasopharyngeal angiofibroma was removed and concluded that the endoscopic approach after embolization is safe, effective, and associated with fewer complications; it is thus ideal for tumors at initial stages. In our series, the mean duration of surgery for endoscopy was 107.7 minutes; for open procedures it was over 200 minutes (203 minutes for degloving, and 270 minutes for transpalatine surgery). Our findings corroborate these findings; our mean duration for endoscopy was 130 minutes, and for open surgery it was 270 minutes, suggesting that the latter could cause more morbidity.
Among our patients, only one of nine patients that required blood transfusion had undergone endoscopic surgery. We found no relationship as to which would be the best technique for a given stage when analyzing the need for blood transfusions and tumor staging; the patients were distributed non-homogeneously within both surgery groups.
A relatively low blood loss during endoscopic procedures may occur due to the careful nature of dissection. Even small amounts of bleeding limit the surgeon's endoscopic view, so special attention should be given to hemostasis for satisfactory results. Furthermore, most of the blood loss in open approaches occurs because of incisions and osteotomies for surgical access5.
Pryor et al.5 studied 54 patients in which nasopharyngeal angiofibromas were removed; open surgery was done in 49 patients and endoscopic surgery was done in five patients. The authors found that there was less intraoperative bleeding, a shorter hospital stay, and fewer complications and recurrences in patients that underwent endoscopic surgery. The authors also suggested that tumors involving the ethmoid, the maxillary sinus, the sphenoid sinus, and stage I and II (Fisch) can be operate by endoscopy. Tumors that involve the orbit or the cavernous sinus require an intra- and extracranial approach.
Midfacial degloving is a reasonable choice for nearly all advanced tumors - Fisch's stage IV - as it is useful for providing a good surgery view during surgery, esthetic results, and lower morbidity; if necessary, it may be combined with other approaches or craniotomy11,12.
The contraindications against endoscopy are stage IV and some stage III nasopharyngeal angiofibromas, which extend to the mid-cranial fossa. It is advantageous to combine midfacial degloving with endoscopy when removing tumors that extend through the anterior portion of the cribbous lamina, and also to eventually correct cerebrospinal fluid leaks13.
Extension of the tumor to the lateral infratemporal fossa, the sellar area, and areas adjacent to the optic nerve is a challenge for endoscopic resection. When tumors invade these areas, the surgical view is limited even in open procedures and tumors are hard to resect. Endoscopy provides lighting, amplification, and multiangle visualization, which may facilitate removal of tumors that are adjacent to these vital areas. Associating open surgery with endoscopy is advantageous in this context, as the benefits of both approaches are explored, and the possibility of tumor recurrence is reduced8,14.
Still, Carrau et al.15 noted that tumors may be treated by endoscopic surgery alone when the pterygopalatine and infratemporal fossae are involved.
This comparison of our findings with the literature aimed to foster debates on this topic. There is no consensus on when to carry out embolism or which is the best surgical approach for each tumor stage.
CONCLUSION
We found that the mean duration of surgery was shorter when endoscopy was the chosen technique compared to open approaches, which is possibly due to lower morbidity of endoscopy. There was less need for blood transfusion in patients that underwent embolization four days prior to surgery.
REFERENCES
1. Gaillard AL, Anastácio VM, Piatto VB, Maniglia JV, Molina FD. A seven-year experience with patients with juvenile nasopharyngeal angiofibroma. Braz J Otorhinolaryngol. 2010;76(2):245-50.
2. Andrade NA, Andrade JSC, Silva PDM, Oliveira VB, Andrade BB. Angiofibroma Nasofaríngeo: Revisão dos Aspectos Genéticos e Moleculares. Int Arch Otorhinolaryngol. 2008;12(3):442-9.
3. Silva-Rueda R, Revollo-Zuñiga F. Manejo endoscópico de recidiva en nasoangiofibroma juvenil. Acta Otorrinolaringol. 2007;35(1):5-13.
4. Andrade NA, Pinto JA, Nóbrega MdeO, Aguiar JE, Aguiar TF, Vinhaes ES. Exclusively endoscopic surgery for juvenile nasopharyngeal angiofibroma. Otolaryngol Head Neck Surg. 2007;137(3):492-6.
5. Pryor SG, Moore EJ, Kasperbauer JL. Endoscopic versus traditional approaches for excision of juvenile nasopharyngeal angiofibroma. Laryngoscope. 2005;115(7):1201-7.
6. Ferreira LMBM, Gomes EF, Azevedo JF, Souza JRF, Araújo RP, Rios ASN. Endoscopic surgery of nasopharyngeal angiofibroma. Braz J Otorhi¬nolaryngol. 2006;72(4):475-80.
7. Douglas R, Wormald PJ. Endoscopic surgery for juvenile angiofibroma: where are the limits? Curr Opin Otolaryngol Head Neck Surg. 2006;14(1):1-5.
8. Cardozo-Trujillo M, Silva-Rueda R, Caraballo-Arias J. Cirugía endoscópica trasnasal en nasoangiofibromas tempranos: reporte de cuatro casos en el Hospital Militar Central de Bogotá. Acta Otorrinolaringol. 2007;35(1):14-9.
9. Yiotakis I, Eleftheriadou A, Davilis D, Giotakis E, Ferekidou E, Korres S, et al. Juvenile nasopharyngeal angiofibroma stages I and II: a comparative study of surgical approaches. Int J Pediatr Otorhinolaryngol. 2008;72(6):793-800.
10. Nicolai P, Berlucchi M, Tomenzoli D, Cappiello J, Trimarchi M, Maroldi R, et al. Endoscopic surgery for juvenile angiofibroma: when and how. Laryngoscope. 2003;113(5):775-82.
11. Yi ZX, Li ZC, Cheng JM, Zhang R, Lin C, Zhou D, et al. Huge nasopharyngeal angiofibroma with intracranial extension: change in the dura mater and choice of surgical management. J Laryngol Otol. 2007;121(11):1108-12.
12. Danesi G, Panciera DT, Harvey RJ, Agostinis C. Juvenile nasopharyngeal angiofibroma: evaluation and surgical management of advanced disease. Otolaryngol Head Neck Surg. 2008;138(5):581-6.
13. Mann WJ, Jecker P, Amedee RG. Juvenile angiofibromas: changing surgical concept over the last 20 years. Laryngoscope. 2004;114(2):291-3.
14. Marshall AH, Bradley PJ. Management dilemmas in the treatment and follow-up of advanced juvenile nasopharyngeal angiofibroma. ORL J Otorhinolaryngol Relat Spec. 2006;68(5):273-8.
15. Carrau RL, Snyderman CH, Kassam AB, Jungreis CA. Endoscopic and endoscopic-assisted surgery for juvenile angiofibroma. Laryngoscope. 2001;111(3):483-7.
1. Undergraduate medical student, Ceara State University (Universidade Estadual do Ceará or UECE) (Undergraduate medical student, Ceara State University or UECE).
2. Medical doctor, residence in otorhinolaryngology, Fortaleza General Hospital (Hospital Geral de Fortaleza or HGF) SESA/SUS (Otorhinolaryngologist, San Dieu Clinic).
3. Medical doctor, residence in otorhinolaryngology, Fortaleza General Hospital or HGF, SESA/SUS (Otorhinolaryngologist, Otos Clinic).
4. Master's degree in otorhinolaryngology, Sao Paulo Federal University (Universidade Federal de São Paulo or Unifesp) (Head of the Head & Neck Surgery Unit, Fortaleza General Hospital, SESA/SUS).
5. Neuroendovascular surgeon (Neuroendovascular surgeon, Fortaleza General Hospital, SESA/SUS).
6. Doctoral degree in public health, Sao Paulo University (Universidade de São Paulo or USP) (Adjunct professor, Health Science Center (Centro de Ciências da Saúde), Ceara State University or UECE.
7. Medical doctor, doctoral degree in otorhinolaryngology, Sao Paulo University or USP. (Tutor of the residency program, Fortaleza General Hospital, SESA/SUS; doctoral student of otorhinolaryngology, Sao Paulo University or USP.
Fortaleza General Hospital (Hospital Geral de Fortaleza).
Send correspondence to:
José Alberto Alves Oliveira
Rua Bruno Freire, n. 600, ap 2201-A, Cidade dos Funcionárioa
Fortaleza - CE. Zip Code: 60824-010
albertiezzi@hotmail.com
Paper submitted to the BJORL-SGP (Publishing Management System - Brazilian Journal of Otorhinolaryngology) on March 4, 2011.
Accepted on November 24, 2011. cod. 7621.