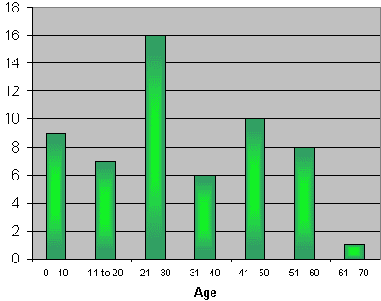Year: 2002 Vol. 68 Ed. 3 - (14º)
Artigo Original
Pages: 388 to 393
Deep neck infections: prospective study of 57 patients
Author(s):
Luiz Ubirajara Sennes(1),
Rui Imamura(2),
Fernando Veiga Angélico Júnior(3),
Lucinda Simoceli(4),
Ronaldo Frizzarini(4),
Domingos Hiroshi Tsuji(2)
Keywords: deep neck space, necrotizing fasciitis, neck space infections
Abstract:
Aim: Since the development of penicillin, the head and neck space infections have become relatively uncommon, although the morbidity remains high. The delayed diagnosis and treatment can cause serious complications as airway obstruction, mediastinitis, sepsis and death. Controversy still exists about choice of antibiotic therapy, diagnosis, and clinical or surgical treatment. The aim of this prospective study is analyze the etiology (associated factors, site of origin and bacteriology), clinical presentation (symptoms, origin, primary and secondary involved spaces and the routes of propagation of the infection), treatment (antimicrobial therapy, corticosteroid, surgical drainage) and complications. Study design: Prospective clinical. Material and method: This prospective series included 58 patients that need hospitalization due to deep neck infection in a two-year period, from December 1997 to December 1999. In all cases there was confirmation of the diagnosis by cervical ultrasound and/or CT scanning. The management consisted of maintenance of an adequate airway and systemic antibiotics. Surgical drainage was performed promptly as required. Bacteriological cultures were done when purulent material was found in spontaneous drainage and during surgical procedures. Results: Thirty patients were males and 28 females. The mean age was 33,4 years. The most common site of infection was the submandibular (46,6%), followed by pterygomandibular (31%), sublingual (29,3%) and parapharyngeal space (20,7%). Thirty-two patients had infection in more than one site. The etiology was odontogenic (40%), suppurative adenitis (13,8%), salivary gland infection (10,3%). Surgical drainage was performed in 72,4% of patients. The association of penicillin and metronidazole was used in 55.2% of cases and clindamicin in 13.8%. The cultures were negative in 38.1%. S. viridans, S. aureus, S. pyogenes, H. influenzae and Peptostreptococcus were isolated. Recovery occurred in 57 patients. One patient died due to mediastinal spread of infection. Two patients had dehiscence of the suture and one had infection recurrence. Conclusion: Deep neck space infections are potential severe disease and a challenging problem in the care of children and adults. An accurate diagnosis based in image studies is related with less complications and sequels. Odontogenic infections are the most prevalent etiology. The association of penicillin and metronidazole still is a cheap and effective treatment to the most cases. The early and wide drainage is the key to infection control, and is related to quickly recuperation and hospital discharge.
![]()
Introduction
Since 1836, when Wilhelm Friedrich von Ludwig30 described the so-called Ludwig Angina, many discussions about clinical manifestations and treatment of deep neck infections and abscesses have taken place.
The progression of diagnostic methods, the development of new antibiotics and more precise and aggressive surgical approaches, together with the prevention of head and neck infections, have provided increased survival of patients and decreased incidence of major complications.
Owing to anatomical characteristics of cervical spaces, despite the use of appropriate antibiotics, deep cervical infections disseminate through the neck fascia and originate abscesses, involving vital structures such as the upper airways, neck viscera and thoracic organs.
In addition to local manifestations that result from the compressive effect of the abscess or the inflammation leading to the development of edema, irritative muscle spasms, and obstruction of the aerodigestive tract, there are also systemic abnormalities such as fever, lethargy, sore throat and presence of edema and neck mass.
The present study intended to report the clinical-radiological findings, clinical and/or surgical approach, as well as the evolution of patients with the diagnosis of deep neck infection, comparing to the findings in the literature.
MATERIAL AND METHOD
We studied prospectively 57 inpatients, between January 1999 and January 2001, from the Division of Clinical Otorhinolaryngology, Hospital das Clínicas, Medical School, University of São Paulo, who had diagnosis of deep neck infections (abscess, cellulitis and necrotizing fasciitis). In all cases, we confirmed the diagnosis through physical examination, neck x-ray, ultrasound and/or computed tomography (CT scan). The affected neck spaces were defined by imaging findings (especially from the CT scan) and or/surgical findings. Chest x-ray was always performed to investigate mediastinum enlargement and if there were doubts, we performed chest CT scan.
All patients were hospitalized, we ensured permeability of airways and introduced parenteral antibiotics. The choice of antibiotic was based on primary infectious site and severity of the case.
Corticosteroids were used for an average period of 72 hours, provided that there were no contraindications, such as diabetes, hormonal dysfunction, non-controlled arterial hypertension, active dyspeptic disease, drug hypersensitivity and immunodepression.
Surgical drainage, in the emergency room or in the hospital setting, was promptly performed if the case severity required it or after imaging tests that showed collection. Aerobe and anaerobe cultures were performed when purulent material was collected by spontaneous drainage or during surgical procedures.
We analyzed the following aspects of the population: age, gender, personal history, diagnosis, etiology, affected neck spaces, number of spaces simultaneously involved, culture microbiology, used antibiotics and progression of patients.
RESULTS
Our sample consisted of 57 patients, divided into 32 male (56.1%) and 25 female (43.9%) subjects. Ages ranged from 2 months to 65 years, mean age of 30.4 ± 17.2 years. The distribution of patients by age may be seen in Graph 1.
As to personal history, two patients (3.5%) were smokers, 1 (1.7%) abused of alcohol and 5 (8.8%) were both smokers and users of alcohol. Diabetes was the most prevalent underlying disease (7 cases - 12.3%), followed by hypertension, schistosomotic hepatic pathology, epilepsy, and users of intravenous drugs (1 case each, 1.7%).
Most of the cases presented neck abscess (87.7%), 5 cases (8.8%) had only cellulitis and 2 cases (3.5%), had necrotizing fasciitis.
The distribution of etiologies of deep neck infections is shown in Table 1.
The development of deep cervical infections is shown in Table 2, being that simultaneous affection of more than one space was noticed in 34 cases (59.6%).
Cultures were conducted with purulent material obtained from 53 patients (93%). Out of the total, 28.3% did not present bacterial growth. Mixed cultures that contained more than one organisms was detected in 11 patients (20.8%).
Table 3 shows the distribution of aerobe and anaerobe microorganisms isolated in the culture of the material collected from the 53 patients.
The antibiotic therapy adopted may be seen in Table 4.
Fifty-four patients (94.8%) improved the clinical picture, with no complications. Only 2 patients (3.4%) presented dehiscence of the suture during treatment and 1 (1.7%) died of mediastinitis.
Graph 1: Distribution of age range of patients.
Table 1. Distribution of etiologies of deep neck infections.
Etiology / N (%)
Odontogenic infections - 24 (42.1%)
Tonsillitis - 10 (17.5%)
Post-UAI lymphadenitis - 9 (15.8%)
Undefined cause - 5 (8.8%)
Skin infection - 3 (5.3%)
Foreign body intake - 2 (3.5%)
Submandibulitis - 1 (1.7%)
Infected branchial cyst - 1 (1.7%)
Parotiditis - 1 (1.7%)
Tumor Necrosis - 1 (1,7%)
Total - 57 (100%)
Table 2. Distribution of affected deep neck spaces.
Spaces Affected / N (%)
Submandibular - 33 (57.9%)
Sublingual - 15 (26.3%)
Parapharyngeal - 14 (24.6%)
Mastication - 14 (24.6%)
Submentonian - 8 (14%)
Parotid - 7 (12.3%)
Anterior Visceral - 7 (12.3%)
Peritonsillar - 4 (7%)
Buccal - 4 (7%)
Retropharyngeal - 4 (7%)
Pre-epiglottic - 2 (3.5%)
Infratemporal - 2 (3.5%)
Canine - 2 (3.5%)
Carotid - 2 (3.5%)
Mediastinum - 1 (1.7%)
Perivertebral - 1 (1.7%)
Table 3. Bacteria isolated in the culture of material collected from the infections.
Aerobe / N (%)
Streptococcus viridans - 22(41.5%)
Staphylococcus aureus - 11(20.7%)
Haemophilus influenza - 2 (3.8%)
Streptococcus pyogenes - 1 (1.9%)
Streptococcus epidermidis - 1 (1.9%)
Streptococcus b-hemolytic - 1 (1.9%)
Klebsiella pneumoniae - 1 (1.9%)
Corynebacterium sp - 1 (1.9%)
Neisseria sp - 1 (1.9%)
Acinetobacter baumanii - 1 (1.9%)
Pseudomonas aeruginosa - 1 (1.9%)
Total - 43 (81.1%)
Anaerobes / N (%)
Peptostreptococcus micros - 3 (5.7%)
Peptostreptococcus sp - 2 (3.8%)
Peptostreptococcus magnus - 1 (1.9%)
Peptostreptococcus anaerobius - 1 (1.9%)
Prevotella oris - 1 (1.9%)
Prevotella corporis - 1 (1.9%)
Prevotella intermadia - 1 (1.9%)
Prevotella melaninogenica - 1 (1.9%)
Clostridium bifermentalis - 1 (1.9%)
Lactobacillus sp - 1 (1.9%)
Fusobacterium varium - 1 (1.9%)
Propiniumbacterium acnes - 1 (1.9%)
Total - 15 (28.3%)
Table 4. Antibiotics used for management.
Antibiotics / N (%)
Crystalline Penicillin and Metronidazole - 31 (54.4%)
Clindamicyn - 8 (14%)
Penicillin - 3 (5.3%)
Clindamicyn and 3rd generation Cephalosporin - 2 (3.4%)
Crystalline Penicillin and Chloramphenicol - 2 (3.4%)
Cephalotin - 2 (3.4%)
Oxacillin - 1 (1.8%)
Chloramphenicol - 1 (1.8%)
Oxacillin and Chloramphenicol - 1 (1.8%)
Crystalline Penicillin and 3rd generation Cephalosporin - 1 (1.8%)
Oxacillin and Metronidazole - 1 (1.8%)
Metronidazole and 3rd generation Cephalosporin - 1 (1.8%)
Clindamicyn and Amikacin - 1 (1.8%)
Vancomicyn and Imipenem - 1 (1.8%)
Crystalline Penicillin, Metronidazole and Amikacin - 1 (1.8%)
Total - 57 (100%)
Discussion
Clinical Presentation
Classically speaking, neck space infections may affect patients at any age range, regardless of gender. Our study found a higher incidence among adults aged on average 30.4 years, ranging from 2 months to 65 years, as shown by Graph 1. Both genders were affected, with a slight predominance of male subjects (56.1%).
A number of other causes have been associated with neck spaces, including sialoadenitis, aerodigestive tract damage during orotracheal intubation and upper digestive endoscopy, foreign body ingestion29, penetrating traumas and/or neck concussion and neck congenital damage, especially branchial cleft cysts, which are associated with recurrent neck infections22.
We noticed that odontogenic focus was the main factor responsible for infections (42.1% of the cases), followed by tonsillar foci (17.5%) and abscessed neck lymphadenitis (15.8%). These three foci amounted to 75.4% of all our cases.
According to literature reports, the infections are associated with dental and pharyngeal foci and most of the patients report to the doctors history of upper airway infection or dental infection or manipulation before the onset of the clinical picture. However, some patients do not associate it with triggering factors.
Among the other causes, we noticed foreign body intake in 3.5% of the cases, submandibulitis in 1.7%, branchial cleft cyst in 1.7%, tumor necrosis in 1.7% and skin infection in 5.3% of the cases, in accordance with the literature27. Undefined causes were noticed in only 8.8% of the cases, which is below the figures reported by the literature, which range from 25%24 to 37.5%6. This issue could be explained by the fact that we had only included inpatients because cases of lymphadenopathies are difficult in defining the etiology and they are normally treated in outpatient settings.
Neck infections may disseminate local-regionally through different routes, such as the hematogenic and lymphatic ones, but especially through direct extension (contiguity) by dissecting the fascia, reaching and involving multiple neck spaces15.
Considering anatomical regions between the spaces and facial structures and the highest incidence of dissemination by direct extension, the involvement of specific spaces is typical, according to the focus that originated the infection15. Odontogenic infections affect the submandibular, submentonian and sublingual spaces, and later the mastication and parapharyngeal spaces12. The latter, owing to its location in the merge of different spaces, is frequently infected when the foci are pharyngeal, especially by tonsillitis and other upper airway infections.
As previously mentioned, the most common infectious focus in the present study was the odontogenic focus (42.1%), followed by tonsillitis (17.5%) and post-upper airway infection (UAI) lymphadenitis (15.8%). As reported in the literature, we observed that the most frequently affected spaces were submandibular (57.9%), sublingual (26.3%), parapharyngeal ((24.6%), mastication (24.6%), submentonian (14%), peritonsillar (7%) and buccal (7%).
These findings are important to guide the clinical and radiological investigation of patients to predict complications and to plan surgical approach.
Microbiology
Some authors reported that the cases that have high prevalence of bacteria in direct bacterioscopy (gram) but have negative culture may reflect the presence of anaerobes10.
Microbiology of deep neck spaces is characterized by a predominance of gram positive bacteria, especially of Streptococcus and Staphylococcus aureus19 species, but the identification of anaerobic germs in cultures emphasizes the importance of polymicrobial infections9.
We collected material for culture in 53 cases and out of this total, 28.3% did not present bacterial growth. There are a number of causes of absence of growth, such as previous antibiotic therapy, inappropriate collection and transport or delay in taking the material to the lab. In case of more sensitive bacteria, the culture may be negative. The main isolated bacteria in our cultures were gram positive, with 41.5% Streptococcus viridans, 20.7% Staphylococcus aureus and 5.7% other species of Streptococcus. In 20.8%, cultures had a mixed flora.
Considering only anaerobes, the species of Peptostreptococcus amounted to 13.3% of the cultures and Prevotella, 7.6%, data that are in agreement with the most recent studies published in the literature. As previously reported, these are the most difficult bacteria to isolate because they do not resist to environment exposure.
The presence of gram negative bacteria in the study, especially of H. influenzae (3.8%), K. pneumoniae (1.9%), Corynebacterium sp (1.9%), Neissseria sp (1.9%), Acinetobacter baumanii (1.9%) and Pseudomonas aeruginosa (1.9%), amounting to 13.3 % of the sample, was quite similar to literature reports that mentioned 18% of gram negative bacteria in their samples, especially of H. influenzae. This microorganism may be isolated in post-UAI lymphadenitis, but the other gram-negative organisms are normally found in polymicrobial flora or in patients with underlying diseases.
The association of some systemic diseases and deep neck infections is frequently mentioned, and diabetes is one of the most significant pathologies concerning risk factor for complicated infections and progression to death. Authors such as Lee16 reported greater susceptibility of these patients to gram negative bacterial or Staphylococcus species infections. Seven patients (12.28%) of the present study had previous diagnosis of diabetes and the organisms were isolated in 6 cultures (1 had phlegmon), divided as: Streptococcus viridans (2 patients), S. aureus (1), Klebsiella pneumoniae (1) and mixed flora comprising S. viridans, Peptostreptococcus anerobious and P. micros in 2 patients. The only case of death was part of this group, with mixed flora in the culture.
Users of intravenous drugs formed a specific group that presented higher incidence of gram positive infections, especially by Staphylococcus and Streptococcus strains28. We observed only one patient who was user of drugs whose culture revealed S. viridans, H. influenza and Peptostreptococcus.
Therapeutic Approach
The basic principles of treatment of deep neck space infections are maintenance of upper airways, antimicrobial therapy and surgical drainage.
Maintenance of upper airways is a treatment priority and in some situations such as in abscesses involving different cervical spaces with reduction or occlusion of air spaces, orotracheal intubation, tracheostomy or cricothyroidectomy should be considered20.
Antimicrobial therapy should be introduced at the time of clinical assessment of patients and our choice was based on wide coverage of the main bacteria involved, gram positive and anaerobes, for intravenous administration. The combination of penicillin and metronidazole was used in 54.4% of the cases. The adjustment of the drugs, if necessary, was performed after the result of the antibiogram study, directing treatment to the specific coverage of the isolated bacteria.
Most of the literature studies mention the use of wide-spectrum antimicrobial combinations to cover gram positive, gram negative and anaerobe microorganisms as the initial treatment choice6.
However, our data showed that initial coverage of gram negatives would be beneficial only in 12.3% of the cases, considering that they are normally isolated in multiple flora.
Taking into account the most frequent etiologies of S. aureus, hemolytic streptococcus and anaerobes the literature recommends the use of penicillin for gram positive and anaerobe, Clindamicyn or metronidazole for anaerobes and 3rd generation cephalosporin or aminoglycosides for gram negatives.
In our study, the association of 2 antimicrobial agents was the choice in 72% of the patients and the combination we used the most was crystalline penicillin and metronidazole. This combination may be replaced by clindamicyn with advantages, since it presents better coverage of S. aureus, has good penetration in the bone and may be orally administered. Although its spectrum for anaerobes is lower than that of metronidazole., it has good coverage of anaerobes involved in neck infections. Three antibiotics were used only in 1 patient (1.7%) and in 26.2% of the cases, we used only one antibiotic.
The literature brings about questions concerning the appropriate moment to surgically approach these infections. Some authors8 advocated early surgical intervention even in cases in which clinical-tomographic findings are compatible only with cellulitis, without purulent collection, but most believe in a more traditional approach, indicating surgery only when there is evidence of liquefaction amidst tissues7. In our study, 87.7% of the patients presented clinical and/or tomography findings of purulent collection and 2 patients (3.5%), of necrotizing fasciitis, who were surgically treated. Only in 5 patients (8.8%), the infectious process was diagnosed as cellulitis, which were clinically treated. We should highlight, however, that cellulitis may progress to abscesses, requiring surgical drainage.
Our results were satisfactory, with cure and no sequelae, in 94.8% of the cases. Two patients had surgical wound dehiscence and one died of necrotizing fasciitis complicated by mediastinitis. Previous studies about deep neck infections reported higher incidence of complications, about 19%26, emphasizing the cases of necrotizing fasciitis, whose mortality rate ranged from 38% to 50%13, requiring repetitive debridment and prolonged antibiotic therapy.
Other complications such as aspiration pneumonia, acute myocardium infarction, diabetic ketoacidosis, carotid artery rupture, septicemia and multiple organ failure were also reported11, 14, 17. Special attention should be given to vertebral complications, observed in 2 cases, especially vertebral osteomyelitis, intravertebral disk infection (diskitis) and epidural abscess of the cervical spine, normally consequence of the direct extension of the infection through the pre-vertebral fascia, since these pictures amount to low percentages of complications, but represent high morbidity rates to patients23, 25.
Conclusion
Aggressive treatment of deep neck infections, with maintenance of the airways, antibiotic therapy and early surgical drainage reduce complication rate. The coverage for gram positives and anaerobes is enough in most cases, which should be extended in severe cases or when the primary focus justifies the incidence of gram negatives. Radiological assessment supports differential diagnosis between cellulitis and abscess and guide management.
REFERENCES
1. Becker M, Zbären P, Hermans R et al. Necrotizing fasciitis of the head and neck: Role of CT in diagnosis and management. Radiology 1997;202:471-476.
2. Brook I. Microbiology of abscesses of the head and neck in children. Ann Otol Rhinol Laryngol 1987;96:429-433.
3. Brook I. Diagnosis and management of anaerobic infections of the head and neck. Ann Otol Rhinol Laryngol 1992;101:9-15.
4. Choi SS, Vezina G, Grundfast KM. Relative incidence and alternative approaches for surgical drainage of different types of deep neck abscesses in children. Arch Otol Head Neck Surg 1997;123(12):1271-1275.
5. Delap TG, Grant WE, Dick R, Quiney RE. Retropharyngeal abscess - an unusual complication of anorexia nervosa. Journ Laryngol Otol 1996;110:483-484.
6. El-Sayed Y, Al Dousary S. Deep-neck space abscesses. The Journal of Otolaryngology 1996;25(4):227-233.
7. Endicott JN, Nelson RJ, Saraceno CA. Diagnosis and management decisions in infections of deep fascial spaces of the head and neck utilizing computerized tomography. Laryngoscope 1982;92:630-633.
8. Flynn TR. Odontogenic infections. Oral maxillofac Surg Clin North Am 1991;3:311-329.
9. Gianoli GJ, Espinola TE, Guarisco JL, Miller RH. Retropharyngeal space infection: changing trends. Otol-Head and Neck Surgery 1991;105(1):92-100.
10. Gidley PW, Ghorayeb BY, Stiernberg CM. Contemporary management of deep neck space infections. Otol-Head and Neck Surgery 1997;116(1):16-22.
11. Kaplow HJ, Eichel BS. Deep neck infections. Otolaryngology 1980;3:14-17.
12. Kim HJ, Park ED, Kim JH, Hwang EG, Chung SH. Odontogenic versus nonodontogenic deep neck space infections: CT manifestations. Journal of Computer Assisted Tomography 1997; 21(2):202-208.
13. Krespi,YP, Lawson W, Blaugrund SM. Massive necrotizing infections of the neck. Head Neck Surg 1981;3:474-481.
14. Langerbrunner DJ, Dajani S. Pharyngomaxillary space abscess with carotid artery erosion. Arch Otol 1971;94:447-452.
15. Lazor JB, Cunningham MJ, Eavey RD, Weber AL. Comparison of computed tomography and surgical findings in deep neck infections. Otol-Head and Neck Surgery 1994;111(6):746-750.
16. Lee KC, Tami TA, Echaves M, et al. Deep neck infections in patients at risk for acquired immunodeficiency syndrome. Laryngoscope 1990;100:915-919.
17. Levitt GW. The surgical treatment of deep neck infections. Laryngoscope 1971;81:403-411.
18. Miller WD, Furst IM, Sandor GKB, Keller MA. A prospective, blinded comparison of clinical examination and computed tomography in deep neck infections. Laryngoscope 1999;109:1873-1879.
19. Nagy M, Pizzuto M, Backstrom J, Brodsky L. Deep neck infections in children: A new approach to diagnosis and treatment. Laryngoscope 1997;107:1627-1634.
20. Nagy M, Bacstrom J. Comparison of the sensitivity of lateral neck radiographs and computed tomography scanning in paediatric deep-neck infections. Laryngoscope 1999;109:775-779.
21. Nicklaus PJ, Kelley PE. Management of deep neck infection. Pediatric Clinics of North America 1996;43(6):1277-1297.
22. Nusbaum AO, Som PM, Rothschild MA, Shugar JMA. Recurrence of a deep neck infection - A clinical indication of an underlying congenital lesion. Arch Otol Head Neck Surg 1999;125(12):1379-1382.
23. Piccolo R, Passanisi M, Chiaramonte I, et al. Cervical spinal epidural abscesses - A report on five cases. Journal of Neurosurgical Sciences 1999;43(1):63-67.
24. Pontell J, Har-El G, Lucente FE. Retropharyngeal abscesses: Clinical review. ENT 1995;74(10):701-704.
25. Saban KL, Ramsis FG. Spinal cervical infection: A case report and current update. Journal of Neuroscience Nursing 1998;30(2):105-115.
26. Sethi DS, Stanley RE. Deep neck abscess - changing trends. Journal Laryngol Otol 1994;108:138-143.
27. Szuhay G, Tewfik TL. Peritonsillar abscess or cellulitis? A clinical comparative pediatric study. The Journal of Otolaryngology 1998;27(4):206-212.
28. Tom MD, Rice DH. Presentation and management of neck abscess: a retrospective analysis. Laryngoscope 1988;98:877-881.
29. Tsai YS, Lui CC. Retropharyngeal and epidural abscess from swallowed fish bone. Am J of Emerg Med 1997;15(4):381-382.
30. Tschiassny K. Ludwig's Angina - A surgical approach based on anatomical and pathological criteria. Ann Otol Rhinol Laryngol 1947;56:937-945.
[1] Full Professor, Discipline of Otorhinolaryngology, Medical School, University of São Paulo.
[2] Assistant, Ph.D., Division of Clinical Otorhinolaryngology, Hospital das Clínicas, Medical School, University of São Paulo.
[3] Post-Graduate studies under course, Discipline of Otorhinolaryngology, Medical School, University of São Paulo.
[4] Resident physician, Discipline of Otorhinolaryngology, Medical School, University of São Paulo.
Study conducted in the Division of Clinical Otorhinolaryngology, Hospital das Clínicas, Medical School, University of São Paulo.
Study presented at II Congresso Triológico de Otorrinolaringologia, held in Goiânia on August 22 - 26, 2001.
Free Paper awarded with a special citation.
Address correspondence to: Lucinda Simoceli - Rua Mourato Coelho, 756 Ap: 65 - Pinheiros - São Paulo - 05417-001 - Tel. (55 11) 9383.6673 Fax: (55 11) 3280.0299 - E-mail: lsimoceli@bol.com.br