Year: 2002 Vol. 68 Ed. 3 - (10º)
Artigo Original
Pages: 355 to 360
Search of the nystagmus/positional vertigo and electronystagmographic evaluation in a group of diabetics Mellitus type I
Author(s):
Lílian P. Scherer(1),
Fga. Marilda B. Lobo(2)
Keywords: diabetes Mellitus, electronystagmographic, irritable vestibular sindrome, vertigo, labyrintholgy
Abstract:
Introduction: According to the Brazilian Society of Diabetes, there are 5 million diabetics in Brazil. In the state of Rio Grande do Sul only, there are, probably, 400.000 diabetics23,27. This group of people can present vestibular alterations. Aim: This article had the objective to contribute with the searches to help improve the quality of life of the diabetics and to search the nystagmus/ positional vertigo and electronystagmographic evaluation, and to identify possible alterations. Study design: Prospective clinical. Material and method: 12 Diabetics Type I, insulin dependents, between 12 and 27 years old, and members of Associação Riograndense de Apoio aos Diabéticos (ARAD). To observe the vestibular reactions, we did a specific interview, otoscopy, tympanometric, search of the Nystagmus/ Positional Vertigo and Electronystagmographic Evaluation in the selected sample. Results: We verified Periferic Irritable Vestibular Syndrome in 75% of the altered results. 62,5% in this group didn't present otoneurologyc symptom. Conclusions: We note that the effect of Diabetes Type I in the vestibular function may be taken into consideration like any other usual complications, since the same may occur in asymptomatic patients, and also because with the early etiologic diagnostic it is possible to help in the prevention of this disease's complications.
![]()
Introduction
Diabetes Mellitus is a genetically determined chronic disorder that results from the affection of glucose metabolism caused by absolute or relative absence of insulin3, 28.
Insulin is a protein produced by the pancreas, responsible for the consumption and metabolism of glucose in body cells, which aim at producing energy23.
Type I diabetes is normally manifested before 15 years of age and it is characterized by quick onset and the presence of classical symptoms29.
Type II diabetes is normally noticed after the age of 40. Symptoms are less marked and they may remain undetected for a long time23, 27, 29.
There are currently about 135 million people with diabetes in the world. It is estimated that in Brazil there are about 5 million people with the pathology. In Rio Grande do Sul state, there are approximately 400,000 subjects who have the syndrome27.
Significant advances in the definition of diagnostic criteria and the knowledge of new management strategies have been reported in recent years. However, many of these transformations are not sufficiently consolidated yet, hindering the management of diabetic patients by general practitioners who have to make decisions about the best modern therapeutic methods.
Glucose metabolism has great influence over the inner ear metabolism. Bittar et al.4, Ferreira Jr. et al.13 and Ramos et al.25 stated that the inner ear is one of the most active metabolic organs in the body. However, the structure does not have stored energy reserve, and minor blood glucose variations influence its functioning, causing balance disorders. According to Mor et al.21, the space between bone and membranous labyrinth in the inner ear is filled with perilymph, which is rich in sodium, whereas the inner portion of the membranous labyrinth is filled with endolymph, which is rich in potassium.
According to Ferreira Jr. et al.13, in metabolic disorders, the potassium from the endolymph tends to invade the perilymph and the sodium does the inverse movement. This mechanism is responsible for vertigo, tinnitus, hearing loss and ear fullness.
For the inner ear to properly operate, it is necessary to have a balance of insulin level and glucose intake. In cases of diabetes mellitus, there is blood glucose but the substance is not capable of coming into the inner ear because of insulin deficiency13, which could explain the statement made by Fangchao12, who said that diabetic patients that use insulin have better hearing thresholds than those who do not, referring that the use of insulin could limit the progression of the hearing loss.
Perez et al.24 believed that there was a functional deficit of the vestibular organ in diabetic metabolic status.
OBJECTIVES
The present study intended to:
· Investigate nystagmus and positional vertigo and electronystagmography in subjects with type I diabetes mellitus;
· Identify possible observable abnormalities.
Material AND METHODS
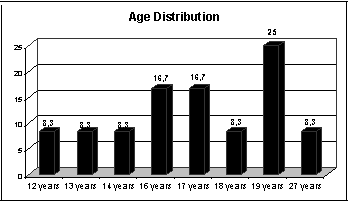
We conducted an observation of a transversal contemporary descriptive group. The sample included 12 subjects members of the Rio Grande do Sul Diabetes Support Association who were users of insulin and had type I diabetes mellitus. We did not assess patients with type II diabetes to rule out associated factors, such as age and obesity, in accordance with the studies by Biurrun et al.5, and there were 58.3% male and 41.7% female subjects.
Data collection took place between August 10 and October 5, 2001. The control group was not used, because according to Caovilla et al.7, glucose and insulin curves are considered essential to confirm abnormality by hyperinsulinemia, thus necessary to exclude glucose metabolic abnormalities in subjects considered healthy and asymptomatic.
No subjects were excluded due to external or middle ear affections, such as malformation, otitis, wax plug, and tympanic perforation.
The inclusion criteria of the study were subjects with type I diabetes mellitus, adolescents and adults up to 40 years of age. Subjects older than 40 years were excluded to prevent aging co-morbidities, such as presbyvertigo or presbytaxis (elderly imbalance). These pathologies may be caused by degenerative processes of the peripheral and central vestibular system, reducing their skills to match visual, proprioceptive and vestibular signs and to modify adaptation reflexes26. We also excluded subjects who refused to commute to the Lutheran University of Brazil to undergo the tests.
In order to check the vestibular responses in the studied group we performed the following:
· Specific history to guide the examiner6, and to exclude subjects who had other pathologies that could interfere in the results of the tests;
· Otoscopy: to visualize the placement of the immittance probe and to check the presence of malformation, wax plug or blood;
· Tympanometry, which intended to identify middle ear abnormalities, such as otitis and tympanic perforation;
· Nystagmus and Positional vertigo tests at supine position, right and left lateral positions, supine position with pending head, supine position with head pending to the right, to the left and seating position. During the investigation, the subjects were placed on the examination table. These tests were performed because according to Bolsen and Torres6, the presence of nystagmus and/or vertigo at any position indicates irritable abnormality, although it does not define location, and Caovilla et al.8 stated that the presence of vertigo and/or positional nystagmus is very frequent in patients with vestibular pathologies.
· Electronystagmography: Calibration, spontaneous nystagmus with opened and closed eyes, semi-spontaneous nystagmus to the right and left, pendulum tracking, optokinetic nystagmus to the right and left performed with seated patients; recalibration, pre-caloric nystagmus (closed eyes) and post-caloric nystagmus (closed eyes and opened eyes to investigate EIFO) performed with the patients lying down at position I of Brünnings - head at 30o angle of the horizontal plane15, 16. To perform electronystagmography, we cleaned the skin with alcohol before the placement of electrodes: one on the right external canthus, one on the left external canthus and one on the face (grounding electrode). The subjects were placed as to be 1.5 meter far from the biological control unit (for calibration and recalibration), so that the adjustment of the recorder of the electronystagmographer was as follows: 1 degree of eye deviation represented 1 mm of deviation of the recorded on the paper21 in millimeters. We used air for caloric stimulation at 20o C (cold) and 42o C (hot) at 80 seconds, in the following order: cold in the left ear, cold in the right ear, hot in the right ear, hot in the left ear15. The recording time (closed eyes) was 30 seconds, started 10 seconds after the end of caloric stimulation, so that we could analyze the period with greater responses6. Within this period, we selected three episodes of nystagmus to be measured and calculated the arithmetic mean of VACLs 21.
We used the following materials: conducting gel, gauze, alcohol, adhesive tape, Biological Control Unit for Nystagmography Berger Model CB-115 (biological cross), Optokinetic Stimulator Berger Model TB - 113, Vecto-nystagmographer Berger Model VN - 316, paper roll in millimeters and Otocalorimeter Berger Model AR - 314.
At the end of the assessments, we conducted qualitative assessment of the results (analysis of tracings) and calculations of PDN for optokinetic nystagmus, and PDN and PL (if arreflexia, hypo or hyper-reflex were not present) for post-caloric nystagmus. For the analysis of the results, we used the values proposed by GANANÇA et al.15 for specification of results for air caloric tests for PDN and PL.
Results AND DISCUSSION
In 100% of the studied sample, we did not observe the isolated presence of positional nystagmus. These data are in accordance with Silva et al.26 who stated that there are no positional nystagmus in subjects with metabolic disorders. Conversely, Biurrun et al.4 reported the presence of positional nystagmus in 26.1% of the subjects with type I diabetes mellitus studied by them. 16.7% of the subjects manifested the presence of paroxysmal and fatigable vertigo, without nystagmus at head pending to the right and left positions.
Ganança et al.15 confirmed that positional vertigo indicated disorders normally related to peripheral vestibular system.
Caovilla et al.8, Ganança et al.14 and Bolsen and Torres6 referred that the presence of vertigo at any position during the test indicated irritable vestibular abnormality without definition of location.
One hundred percent of the studied subjects showed regular tracing upon calibration. These data are in agreement with the data reported by Bolsen and Torres6, who reported that regular tracing - repetitive rectangular - is the expected type at eye calibration in normal subjects or in those with peripheral vestibular disorders; Ganança et al.14, which reported abnormalities especially in vertigo spells and central vestibular syndromes; Silva et. al.26, which said that saccadic movements in subjects with labyrinthopathy of metabolic origin normally do not present abnormalities, and Almeida2, which found regular calibration in 100% of the subjects studied by her who had metabolic abnormality of thyroid origin.
We did not observe the presence of opened eyes spontaneous nystagmus in 100% of the sample, which indicates normal results, since according to Lopes Filho and Campos20, opened eyes spontaneous nystagmus is always pathological. These data are in agreement with Silva et al.26, which reported that spontaneous nystagmus are normally absent in subjects with labyrinth pathologies of metabolic origin. However, the data disagreed from the findings by Almeida2, who found opened eyes spontaneous nystagmus in 11 of the 25 subjects with thyroid metabolic disorder. To Bolsen and Torres6, in peripheral syndromes, opened eyes spontaneous nystagmus is only manifested in labyrinthic crisis, which did not occur during the collection of the present data.
One hundred percent of our sample did not show spontaneous nystagmus. These data are in agreement with Caovilla et al.9 and Silva et al.26, who reported that spontaneous nystagmus do not normally manifest in subjects with metabolic labyrinthic pathologies, and Bittar et al.4 that said that caloric test is the only test that is abnormal in diabetic patients. The present data were not in agreement with the findings by Almeida2, which detected the presence of closed eyes spontaneous nystagmus in 75% of their sample who had thyroid metabolic disorders, and Biurrun et al.5 who studied electronystagmographic abnormalities in subjects with type I diabetes mellitus reported that 15.2% of the sample manifested spontaneous nystagmus. Those authors did not report if nystagmus was present with opened or closed eyes, or both.
In 100% of the studied sample, we did not observe the presence of semi-spontaneous nystagmus. None of the studied subjects was experiencing a labyrinthic crisis during data collection, since Lopes Filho and Campos20 reported that opened eyes semi-spontaneous nystagmus is present only during peripheral syndrome crisis.
Bittar et al.4, Ramos et al.25, Biurrun et al.5 and Almeida2 did not report such test in their studies.
In 58.3% of the sample we noticed type I pendulum tracking and in 41.7% of them there was type II, which was characterized as normal or off-crisis peripheral syndrome for Lopes Filho and Campos20, Bolsen and Torres6 and Mor et al.21. These data are in accordance with Silva et al.26 who stated that pendulum tracking does not reflect abnormalities in subjects with metabolic labyrinthic pathology, and with Almeida2, who found types I and II pendulum tracings in 25 studied subjects with thyroid metabolic dysfunction.
One hundred percent of the sample presented symmetry at optokinetic assessment. These data are in agreement with Almeida2 results, which found 100% symmetry in thyroid metabolic dysfunction. Silva et al.26 also confirmed that optokinetic nystagmus is normally normal in subjects with metabolic labyrinthic pathology.
In our sample, 100% of the patients did not present nystagmus at the pre-caloric test.
Ganança et al.15, Bolsen and Torres6 and Mor et al.21 suggested the investigation of pre-caloric test nystagmus before the thermal stimulation to check the influence of nystagmus in the results of the caloric test.
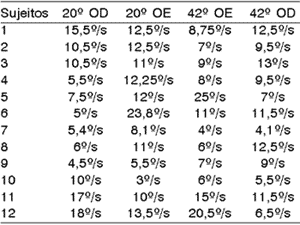
One hundred percent of the patients presented normal reflex upon the analysis of VACL in absolute values. To Ganança et al.15, normal reflex comprises VACL values between 2 and 28º/s; to Bolsen and Torres6, between 3 and 36º/s.
Table 1.1. Values of VACL of caloric test of 12 studied subjects.
These data are in disagreement with Almeida2, who found hyper-reflex in 20% of the thyroid metabolic abnormal patients. Aantaa and Lehtonen1 also reported the predominance of hypo-reflex in diabetic subjects, as well as Ramos et al.25, which reported high incidence of labyrinthic hyper-reflex in subjects with body and/or hearing disorders associated to insulinemia. Cojazzi11 apud Biurrun et al.5 reported hyper-reflex as the main finding and Biurrun et al.5, found hypo-reflex in 10 of the 46 studied subjects who had type I diabetes mellitus.
Table 1.2. Caloric test data.
Graph 1.1 Age Distribution
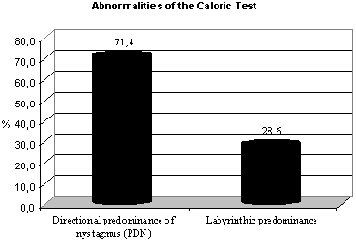
Graph 1.2 Abnormalities of caloric test.
These data are in agreement with the findings of Silva et al.26 which reported that in air caloric test the responses found in subjects with labyrinthic pathology of metabolic origin are directional predominance of nystagmus (PDN), showing irritable compromise of the structure. Caovilla et al.9 found that one of the main findings was directional predominance of nystagmus and Biurrun et al.5, reported the presence of directional predominance of nystagmus in patients with diabetes mellitus. Our data are in disagreement with those described by Camiasca10 apud Biurrun et al.5 who found labyrinthic predominance in 45% and directional predominance of nystagmus in 18.7% of the subjects with type I diabetes. The same applies to Cojazzi11 apud Biurrun et al.5, which reported only some cases of directional predominance of nystagmus in their studies, and Bittar et al.4, which reported that in some cases, electronystagmography may have normal results in diabetic patients.
Among the subjects of our sample, 33.33% presented results within the normal range, whereas 50% had results indicating irritable peripheral vestibular syndrome without definition of location and 16.7% had deficit peripheral vestibular syndrome.
Of the 8 (100%) abnormal tests, 75% had final result of irritable peripheral vestibular syndrome (caused by PDN and positional vertigo) and 25% had deficit peripheral vestibular syndrome (PL). The results we found, indicating 75% of irritable vestibular syndrome, are descriptively relevant according to Gil17, and they confirmed what Silva et al.26 referred upon stating that otoneurological assessment in subjects with metabolic labyrinthic pathology indicates a irritable pattern and metabolic labyrinthic pathologies are characterized by resulting in peripheral vestibular syndrome. Ramos et al.25 also highlighted that there was high incidence of labyrinthic hyper-reflex at caloric tests in subjects who had body and/or hearing complaints associated to insulinemia, whereas Cojazzi11 apud Biurrun et al.5, reported that the main finding was bilateral hyper-reflex. However, the data are in disagreement with Aantaa and Lehtonen1 who reported reduced incidence of caloric reactions in diabetic subjects.
In 87.5% of the cases the responses to electronystagmography were abnormal and in only 12.5% of the subjects the diagnosis was defined based on positional vertigo study.
Even though it was not the purpose of the present study, among the 100% studied subjects, 25% manifested complaints of dizziness in specific situations of hypoglycemia, whereas 75% did not report it, as shown by the graph. One hundred percent of the subjects who manifested this symptom had the final diagnosis of irritable vestibular syndrome at electronystagmography. Among the 100% abnormal results, 62.5% did not report dizziness.
These data are in agreement with Jerger and Jerger18, since the authors believed that approximately 20% of the diabetic patients may complaint of dizziness. Olefsky et al.22, Leher et al.19 and Perez et al.24 believed there was a connection between diet and vertigo complaints. All subjects in the sample had nutritional follow-up. Bittar et al.4, Ramos et al.25 and Ferreira et al.13 stated that since the inner ear does not store energetic reserve, small amounts of glucose variation may influence its functioning. Ferreira Jr. et al.13 related reduction of vertigo episodes to diet counseling and physical activities programs, whereas Ramos et al.25 reported that the diabetic patients that make regular use of insulin have less frequent dizziness, a fact that could explain the small incidence of dizziness complaints in the present sample, since the subjects had type I diabetes mellitus and made regular use of insulin. Silva et al.25 confirmed that most cases of inner ear abnormalities in subjects with metabolic disorders is a result of hypoglycemia. Ferreira Jr. et al.13 found a significant number of subjects with metabolic disorders associated to vertigo and hypoglycemia episodes. Finally, the data also agreed with Perez et al.24 information, which considered diabetes mellitus abnormality to be symmetrical, and that it could cause clinical symptoms in severe cases. The data are in disagreement with Ramos et al.25 and Lopes Filho and Campos20, which stated that DM may or may not be associated to symptoms such as tinnitus, vertigo and dizziness.
The effect of diabetes mellitus should be assessed as the other usual diabetic complications, since the etiological diagnosis may help prevent the complications of the disease. We suggest the conduction of a longitudinal study with a more significant population to confirm the metabolic compromise of the vestibular abnormalities found here.
Conclusion
Based on the collected results, we noticed that in the studied group there were 75% of abnormal results that presented irritable vestibular compromise without defined location and we concluded that the vestibular organ should be included in the list of organs and tissues affected by diabetes mellitus.
ACKNOWLEDGEMENT
We would like to thank the Rio Grande do Sul Diabetes Support Association which was available to support us throughout the study, the volunteer participants of the sample, because without their collaboration it would have been impossible to conduct the present study. To my colleagues Geneci Keller, Gislaine Krause, Raquel Hendges, Tatiane Hackenhaar and Verônica Borges who helped us with data collection. To the Speech and Hearing Pathologist Cíntia de Paula, who supported us whenever needed, and to my dear friend Gisele Lins, who supported me in crucial moments throughout the conduction of the present study. We would also like to thank the Speech and Hearing Pathologist Elisea Meurer, who followed me and believed in my potential and who contributed to my development during academic years, and to the Speech and Hearing Pathologist Marilda Lobo for having shared part of her knowledge with me, which will definitely be a solid ground for the beginning of my professional career.
References
1. Aantaa E, Lehtonen A. Electronystagmographic findings in insulin-dependent diabetics. Acta Otolaryngol (Stockh) 1981;91:15-18.
2. Almeida FS de. Disfunção Metabólica Tireóidea e Otoneurologia. Revista Brasileira de Otorrinolaringologia; jul/ago, 1998. Rio de Janeiro: nº 64:351-8.
3. Bevilacqua F, Bensoussan E, Silva JMJ, Castro FS, Carvalhes LP. Manual de Fisiopatologia Clínica. Rio de Janeiro: Atheneu, 1974.
4. Bittar RSM, Sanchez TG, Santoro PP, Medeiros IRT de. O metabolismo da Glicose e o Ouvido Interno. São Paulo: @rquivos da Fundação Otorrinolaringologia. v.2, jan/fev/mar, 1988.
5. Biurrun O, Ferrer JP, Lorente J, De España R, Gomis R, Trassera J. Asymptomatic Electronystagmographic Abnormalities in Patients with Type I Diabetes Mellitus. Journal for Oto-Rhino-Laryngology and Its Borderlands 1991;53:335-8.
6. Bolsen YAE, Torres MLB. Interpretando a eletronistagmografia e vectonistagmografia na avaliação vestibular. In: Gama, Márcia Regina (Org). Resolvendo Casos em Audiologia. São Paulo: Plexus; 2001.
7. Caovilla HH, Ganança MM, Munhoz MSL, Serafini FF. Síndromes Cocleovestibulares e Hiperinsulinemismo. RBM-ORL Set 1994; 1(2), São Paulo.
8. Caovilla HH, Ganança MM, Munhoz MSL, Silva MLG da. Equilibriometria Clínica. São Paulo: Atheneu, 1999. 158p. vol.1.
9. Caovilla HH, Ganança MM, Munhoz MSL, Silva MLG Da, Frazza MM. O Equilíbrio Corporal e Seus Distúrbios. RBM-ORL Mai/jun 1998;5(3):89-91, São Paulo.
10. Camiasca L. L'esame Dell'apparato Cochleovestibulare nel Diabete Mellito. S SCI Med; 5:45-49, 1950 apud Biurrun O, Ferrer JP, Lorente J, De España R, Gomis R, Trassera J. Asymptomatic Electronystagmographic Abnormalities in Patients with Type I Diabetes Mellitus. Journal for Oto-Rhino-Laryngology and Its Borderlands 1991;53:335-8.
11. Cojazzi L. Le Alterazioni Vestibolare nel Diabete. Arscip S Anna Ferrara 1950;3:76-97 apud Biurrun O, Ferrer JP, Lorente J, De España R, Gomis R, Trassera J. Asymptomatic Electronystagmographic Abnormalities in Patients with Type I Diabetes Mellitus. Journal for Oto-Rhino-Laryngology and Its Borderlands 1991;53:335-8.
12. Fangchao MD. Diabetes and hearing impairment in Mexican American Adults: a population-based study. The Journal of Laryngology and Otology 1998;112:835-9.
13. Ferreira Jr. CA, Guimarães RES, Becker HMG, Silva CDL, Gonçalves TML, Crosara PFTB, Morais MPA de. Avaliação Metabólica do Paciente com Labirintopatia. Belo Horizonte, MG: @rquivos da Fundação Otorrinolaringologia Jan 2000;4:28-32.
14. Ganança MM, Munhoz MSL, Caovilla HH, Silva MLG da. Utilidade Clínica do Exame Otoneurológico. RBM-ORL Abr 2000;57, São Paulo.
15. Ganança MM, Caovilla HH, Munhoz MSL, Silva MLGDa; Frazza MM. Etapas da equilibriometria. In: Caovilla et al. Equilibriometria Clínica. São Paulo: Atheneu; 1999. p. 74-8.
16. Garcia FV, Entrudo A, Ferreira AD, Monteiro MC. O Exame Vestibular Convencional e Seus Limites. Estoril: Rapport do II Simpósio Internacional da Vertigem. Organização do GEV Portugal, 1996.
17. Gil AC. Métodos e técnicas de pesquisa social. 3ª. ed. São Paulo: Atlas, 1999. 206p.
18. Jerger S & Jerger J. Alterações Auditivas: Um Manual para Avaliação Clínica. SP-RJ-BH: Atheneu, 1989.
19. Lehrer JF, Poole DC, Seaman M, Restivo D, Hartman K. Identification and Treatment of Metabolic Abnormalities in Patients With Vertigo. Archives of Internal Medicine 1986 August;146(8):1497-500. American Medical Association.
20. Lopes Filho O & Campos CAH de. Tratado de Otorrinolaringologia. São Paulo: Roca, 1994.
21. Mor R, Fragoso M, Taguchi CK, Figueiredo JF. Vestibulometria & Fonoaudiologia: Como realizar e Interpretar. São Paulo: Lovise; 2001. p.186.
22. Olefsky JM, Farquhar JW, Reaven GM. Reappraisal of the role of insulin in hypertriglyceridemia. American Journal of Medicine 1974:551-560.
23. Oliveira JEP de. http://www.diabetes.org.br/Diabetes, abr 2001.
24. Perez R, Ziv E, Freeman S, Sichel J, Sohmer H. Vestibular End-Organ Impairment in an Animal Model of Type 2 Diabetes Mellitus. Laryngoscope 2001 Jan;111:110-3.
25. Ramos RF, Ramos S, Ganança MM, Albernaz PLM, Caovilla HH. Avaliação Otoneurológica em Pacientes com Labirintopatias e Alterações da Insulinemia. São Paulo: Acta Who 1989 Mai/Ago;8(2):63-6.
26. Silva MLGDa, Munhoz MSLei, Ganança M, Caovilla HH. Quadros Clínicos Otoneurológicos Mais Comuns. Série Otoneurológica. São Paulo, Rio de Janeiro, Belo Horizonte: v. III Ed. Atheneu, 2000.
27. Sociedade Brasileira De Diabetes. http://www.diabetes.org.br/Diabetes/diabet_set.html accessed in April, 2001.
28. Wajchenberg BL et al. Diabetes Melito Insulino-Independente (Tipo I): diagnóstico, etiopatogenia e fisiopatologia. In: Wajchenberg BL. Tratado de Endocrinologia Clínica. São Paulo: Roca, 1992.
29. Wajchenberg BL. Diabetes Mellitus. São Paulo: v. III Ed. Sarvier,1970.
[1] Undergraduate in Speech and Language Pathology and Audiology, Lutheran University of Brazil
[2] Master studies under course in Human Communication, Federal University of Santa Maria - RS.
Affiliation: Lutheran University of Brazil - Canoas - RS
Address correspondence to: Lílian Pacheco Scherer
Tel.: (55 51)3222.6663 or (55 51)982.95190 - E-mail: lilascherer@hotmail.com
This study was approved by the Ethics Research Committee, Course of Speech and Language Pathology and Audiology, Lutheran University of Brazil, according to Resolution 196 CONEP, on July 31, 2001 and presented as final paper at Lutheran University of Brazil, on December 17, 2001.