Year: 2002 Vol. 68 Ed. 3 - (9º)
Artigo Original
Pages: 344 to 352
Transient evoked otoacoustic emissions: tool for early detection of hearing alteration in full-term and preterm neonates
Author(s):
Cristiane Fregonesi Dutra Garcia(1),
Myriam de Lima Isaac(2),
José Antônio Apparecido de Oliveira(3)
Keywords: hearing, prematures, neonates, transient otoacoustic emissions
Abstract:
Introduction: The early diagnostic and intervention in the hearing alterations are of fundamental importance in the infantile development. The register of the otoacoustic emissions has been enlargement indicated for being a fast exam, easily applied. Aim: The objectives of the present study were to value the peripheral hearing function of full term neonate and adequate and small preterm neonate for the gestacional age, by the research of the transient otoacoustic emissions, identifying the prevalence of hearing alterations in this population; to verify the influence from the variable gestacional ages and weight in the moment of the birth, as well as the kinds of treatment, mechanics ventilations, administration of ototoxicity medicines and the permanence in the incubator and to analyze the factors that interfere in the programs of neonatal hearing screening. Study design: Clinical prospective. Material and method: There were appraised 157 children, whose 43 were born full term, 79 preterm adequate to gestacional age and 35 small preterm to gestacional age. It had been observed that premature neonates fail more in the answers from otoacoustic emissions. Results: The prevalence of conductive hearing impairment in the population studied was from 29 ears to 1000 and for the sensory-neural hearing impairment from the 16 to 1000. The low weight children in the birth were the most difficult to be appraised. The transient otoacoustic emissions were observed from the beginning of 27 gestacional weeks old. The kinds of treatments used were factors that influenced negatively in the answers of the otoacoustic emissions in premature groups. Conclusion: The early diagnostic work of the hearing impairment must be objective of the interdisciplinary team - neonatologist, pediatrician, ear/nose and throat doctor, audiologist and speech-language pathologist, nurse and relatives - and must be followed, immediately, by the early interventions programs.
![]()
Introduction
Hearing is one of the senses that reveal important information to human development, especially concerning linguistic and psychosocial aspects. It is an afferent pathway to language acquisition and should mature together with other afferent pathways, efferent pathways and global development. The child deprived of stimulation in the first two years of life will never fully develop his/her language potential, be the deprivation caused by lack of hearing or lack of high quality language experience1.
The first years of life have been considered as the critical period for language and hearing development. Early diagnosis and intervention are essential for the development of children that present auditory abnormalities. Objective tests are more sensitive and specific to detect possible abnormalities, especially in newborns and young children. The recording of otoacoustic emissions has been largely indicated because it is a quick, easy to make test and it may reach a large number of subjects in a short period of time, which is very useful in programs of neonatal hearing screening.
In the year 2000, the members of the Joint Committee on Infant Hearing (ASHA, 2001)2 got together to modify the risk indicators for peripheral and central hearing loss previously reported.
From birth to the 28th day of life, when universal hearing screening is not available, the risk criteria are:
- conditions that require 48 hours or longer admission to the neonatal intensive care unit;
- signs or other findings associated to syndromes that have sensorineural and/or conductive hearing loss;
- family history of sensorineural hearing loss in childhood;
- craniofacial abnormalities, including morphological anomalies of pinna and external auditory canal;
- intra-uterus infections, such as cytomegalovirus, herpes, toxoplasmosis, rubella.
We should also include low birth weight, respiratory distress syndrome, bronchopulmonary dysplasia, and prolonged used of mechanical ventilation.
For children aged 29 days to 2 years, who may present progressive hearing loss, the criteria are:
- parents' complaints about speech, language and hearing development;
- family history of permanent hearing loss in childhood;
- signs or other symptoms associated to syndromes that include sensorineural and conductive hearing loss or auditory tube dysfunction;
- post-natal infections associated to sensorineural hearing loss, including bacterial meningitis;
- intra-uterus infections such as cytomegalovirus, herpes, toxoplasmosis, rubella, syphilis;
- neonatal indicators (hyperbillirubinemia that requires blood transfusion, persistent pulmonary hypertension of the newborn associated to mechanical ventilation, use of extra-corporeal oxygenation);
- syndromes associated to progressive hearing loss (neurofibromatosis, osteoporosis, Usher ataxia);
- neurodegenerative disorders (Hunter's syndrome, sensorial-motor neuropathy, Friedreich ataxia and Charcot-Marie-Tooth syndrome);
- recurrent or persistent otitis media with effusion for at least three months.
Other risk factors for hearing loss, according to the Brazilian context, are children too small for the gestational age, maternal use of drugs or alcohol and incubator length of stay higher than 7 days3. The noise in incubators is source of concern for the development of children's hearing because the equipment is frequently used to assist risk newborns and our neonatal units do not have systematic control and maintenance of the equipment; in addition, there is no special attention from health professionals when handling it during the care directly provided to babies4.
Objective measures of Auditory Brainstem Evoked Potentials (BERA) and otoacoustic emissions (OAE) should be the preference for neonatal screening programs2, 5.
In Brazil, in 1998, GATANU (Group of Support to Universal Neonatal Auditory Screening) was created, comprising speech and hearing pathologist, pediatrician and otorhinolaryngologist, with representation from the National Deafness Institute, Brazilian Society of Otorhinolaryngology, and the Ministries of Education and Health6. In 1999, the Brazilian Committee on Hearing Loss in Childhood was created7 and recommended universal neonatal hearing screening from birth to 3 months of age and if hearing loss was detected, the intervention should start at the age of 6 months, based on the proposal of the international committee2. The committee considered that it is essential to develop a national public health policy that considers early diagnosis of deafness and its intervention, as well as the creation of a program to accomplish such objective, by the joint actions of the Ministries of Health and Education and the committee.
Oliveira8 revealed that the presence of childhood ototoxicity is a polemic issue, indicating the need for further studies performed with OAE and BERA. The author also reported that neonates are less susceptible to ototoxicity than adults, but premature babies are more susceptible than term babies and adults.
It is important to highlight that technological advances have provided increased survival to premature low birth weight newborns; thus, there is a growing interest in studying the development of these children, especially those that required intensive care at birth. They are considered risk children for hearing loss because of the clinical problems they presented and the cooperation of an audiologist is essential to manage this population1.
Early diagnosis of hearing loss is a fundamental strategy to plan and introduce therapeutic measures to prevent complications and improve quality of life. Yoshinaga-Itano 9 discussed the benefits of early identification and intervention, a work performed by a multidisciplinary team. For effective diagnostic services there should be short-term intervention programs, that is, within the first year of life. The role of speech and hearing pathologists and otorhinolaryngologists with the multidisciplinary team is essential because they are responsible for supplying nurses, pediatricians, neonatologists and obstetricians with all the information concerning risk factors for hearing loss and the preventive measures against the noise of the nursery, meet with the parents, organize groups of discussion with parents of high risk babies, discuss cases with the team, make modifications to the environment to improve hearing, visual and behavioral development of babies. An important aspect in the perspective of early diagnosis of hearing loss is the instrument to test the hearing function. OAE objectively assesses cochlear function, specifically the outer hair cells, although it does not quantify the hearing loss. It is present in all ears that have normal hearing, but it is not observed when hearing thresholds are above 20-30dB HL (decibel hearing level). The response will be present only when there is auditory integrity up to the cochlea, or in other words, from the external, through the middle and into the inner ear6, 10, 11, 12, 13, 14, 15.
The measures of OAE are intimately related to the integrity of the outer hair cells and with the non-linear cochlear process. The energy released from the cochlea is transmitted by the ossicle chain and tympanic membrane to the external auditory canal, in which it is recorded as OAE. The components that influence OAE measures are the probe, external and middle ears, cochlea and its fine structures. These elements should work properly for the OAE to be recorded. External and middle ears should be healthy, the probe should be correctly placed in the external auditory canal, environment noise should be controlled, the patient has to be silent (it is important to consider the breathing of newborns, which may be noisy), and there is no need for sedation - these are the characteristics of OAE test8, 11, 13, 15. The first documentation of OAE was made by David Kemp, in 1978, who based his investigation on the model proposed by Gold, in 1948. Otoacoustic emissions are classified into spontaneous - that occur without external stimulation, and evoked - generated by external stimulation. OAE are divided into transient OAE (TOAE), produced by short signs, clicks or tone bursts, Distortion Product otoacoustic emissions (DPOAE), bi-tonal stimulation of specific frequencies in a mathematical relation to combine stimuli; and stimulus-frequency OAE, generated by a continuous pure tone that slowly varies the frequency. Clinical applications of OAE may be divided into three main categories: identification of hearing loss (screening in babies and patients who are difficult to test); monitoring of cochlear function under adverse effects (drugs, noise or degenerative processes); differential diagnosis and special applications (cochlear diagnosis/retrocochlear, special populations)10, 11, 13, 15.
The objectives of the present study were: to assess peripheral hearing function in term newborns and pre-term appropriate for the age (AIG) and small for the gestational age (PIG), by studying OAE; to identify the prevalence of auditory disorders in the studied population; to check the influence of gestational age and weight at birth and types of treatment related to high-tech care, such as mechanical ventilation, use of ototoxic drugs, and length of stay in the incubator; to analyze the factors that interfere in neonatal auditory screening and difficulties found to perform the tests, and the application of a proposed program whose objective is early diagnosis of hearing impairment.
Material and Method
The study was carried out in nurseries of pre-term babies, rooming-in accommodations and ambulatory of Otorhinolaryngology, Hospital das Clínicas, Medical School of Ribeirão Preto, University of São Paulo (HCFMRP-USP).
The reference population for the study was term and pre-term babies seen at the neonatal unit of HCFMRP-USP. We included in our sample babies that met the following criteria:
- parents' consent to participate in the study;
- gestational age below 37 weeks for the studied group and equal or above 37 weeks for the control group;
- good general clinical status.
Data collection took place between February 2000 and March 2001.
We assessed 157 children, 43 of them were term babies (Group I) and 114 were pre-term babies. The 114 children in the study group were subdivided into AIG pre-term babies, comprising 79 children (group II) and PIG pre-term babies, comprising 35 babies (group III). Group I consisted of 25 male children and 18 female children. Weight at birth ranged from 1,450g to 4,455g, average of 3,283g and standard deviation of 639.4g; gestational age ranged from 37 to 41 weeks and 6 days, mean of 39 weeks and 2 days and standard deviation of 1 week and 1 day. Group II consisted of 44 male babies and 35 female babies, weight at birth ranged from 650g to 2,980g, average of 1,785g and standard deviation of 545.7g; gestational age ranged from 27 weeks and 4 days to 36 weeks and 4 days, mean of 33 weeks and 2 days and standard deviation of 2 weeks and 1 day. Group III consisted of 9 male babies and 26 female babies, weight at birth ranged from 620g to 2,380g, average of 1,306g and standard deviation of 426.4g; gestational age ranged from 28 weeks to 36 weeks and 4 days, mean of 32 weeks and 3 days and standard deviation of 5 weeks and 4 days.
The study was performed in two steps. The first one was hearing screening with TOAE, before hospital discharge, as many times as needed and possible and after the discharge, for those who could not be tested during hospitalization. The test was repeated when there were no responses present at TOAE.
The children who failed at that stage were referred, upon discharge, to the second stage of the study, performed in the ambulatory setting. The objective was to come to a conclusion about the peripheral hearing status by using new TOAE tracings and if necessary, DPOAE and BERA to define management of each case.
The independent variables used were defined based on the literature review and the proposition of the Joint Committee on Infant Hearing (ASHA, 2001)2, indicated as risk factor for peripheral and/or central hearing loss (dependent variable):
- use of ototoxic drugs;
- use of mechanical ventilation;
- use of incubator.
The project was submitted to the Ethics Research Committee at HCFMRP-USP, and the study was started after their approval. After thorough and detailed explanation about the nature of the research, objectives, methods, suspected benefits and confidentiality, the mother or accompanying person signed an Informed Consent authorizing the voluntary participation of the baby. Next, we performed an investigation in the medical chart of each subject to learn about identification, risk factors related to family and obstetric procedures, data about the delivery and birth conditions, diagnosis and post-natal therapy.
Based on these procedures, we conducted hearing screening using TOAE. OAE were recorded for both ears, following the method proposed by Bray and Kemp11,13. The equipment used was ILO 288 and ILO 92 - Analyzers of Otoacoustic Emissions (Otodynamics Ltda. Version 4.2 e 5.6, respectively), coupled to a portable microcomputer and the second one, to a conventional computer. We used the software Quickscreener, indicated for neonatal hearing screening.
The first step was to inspect the external auditory canal with the otoscope Heyne, so that we could detect any obstructions in the canal that prevented the effectiveness of the results. The criteria employed to consider presence of TOAE responses were:
- presence of response in 3 out of 5 frequency bands, with amplitude of responses (signal/noise ratio) equal or above 3dbSPL (decibel sound pressure level) and reproducibility equal or above 60% in the bands located at 800 and 1,600Hz;
- response amplitude equal to or above 6dBSPL and reproducibility equal or above 70% in the bands located at 2400, 3200 and 4000Hz;
- general reproducibility equal or above 70%;
- probe stability equal or above 70%.
The second stage was the otologic examination performed by the physician, including external and middle ear conditions assessed by otomicroscopy (microscope DF Vasconcelos, with magnification by ten). Next, TOAE were repeated and if the case presented any alteration, we performed DPOAE and BERA.
For DPOAE, the stimuli used had the same intensity (70dBSPL for F1 and F2 - Frequency), at a ratio of 1.22 for frequencies, F2>F1. The results were interpreted in DP gram, which consisted of variation of fixed frequency and intensity at a pre-defined level13. The parameter used to interpret the results was amplitude of response related to noise equal or higher than 3dB, as of 2000Hz. For BERA, we used the equipment NICOLET CA 2000.
For the statistical analysis of results, we employed the following tests: Kruskal-Wallis, chi-square and Z test. Significance level was fixed at 0.05% or 5% (p<0.05). To check the presence of association between dependent and independent variables, we used odds ratio.
Results
In Group I, with 43 term babies, we observed that of the 86 ears (100%), 76 (88.4%) passed TOAE before hospital discharge. After hospital discharge, six ears (7%) passed and four (4.6%) had no conclusion of diagnosis of peripheral auditory function because they never came to the follow-up visit or were not found any longer. Thus, we observed a total of 95% for "pass" and 5% for not conclusive, referring to the first step of the study.
In Group II, with 79 AIG pre-term babies, we observed that of 158 ears (100%), 115 (72.8%) passed TOAE before hospital discharge and 27 (17.1%) failed. After discharge, 8 ears (5%) passed, two (1.3%) failed and six (3.8%) had no diagnostic conclusion of peripheral hearing status because they did not come back for the follow-up. Thus, we observed a total of 77.8% of "pass", 18.4% of "fail", and 3.8% of "not concluded" referring to the first step of the study.
In Group III, with 35 PIG pre-term newborns, we observed that of the 70 ears (100%), 44 (62.9%) passed TOAE and 12 (17.1%) failed it at hospital discharge. After discharge, 14 ears (20%) passed, and there were no ears of "fail" or "not concluded". We observed a total of 83% for "pass" and 17% for "fail" concerning the first step of the study.
Comparing the types of responses of the three groups before hospital discharge, we observed that the difference between groups I and II, for the "pass" ratio was statistically significant (p=0.0024). The difference between groups I and III for the "pass" ratio was significant (p<0.0001) and the difference between groups II and III was also statistically significant (p=0.0336). As to "fail", in the period of hospital discharge, we observed that groups I and II and I and III had statistically significant difference (p<0.0001). In groups II and III, we did not observe difference in the statistical analysis. For the examined ears after hospital discharge, we observed that for "pass", the difference between groups I and II was not statistically significant (p=0.25). Between groups I and III, the difference was statistically significant (p=0.0047) and between groups II and III, the difference was also statistically significant (p<0.0001).
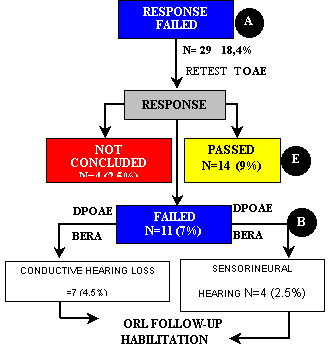
Of the 158 ears of group II, 29 (18.4%) did not pass the first step. TOAE were repeated and we observed that 14 ears (9%) passed, 11 (7%) failed and 4 (2.5%) were interrupted. The four cases not concluded were subjects who could not be found or did not come to complete the tests. Among the 11 ears (7%) that did not present TOAE responses, seven (4.5%) had diagnosis of conductive hearing loss and four (2.5%) of sensorineural hearing loss (Figure 1, Flow Chart 1), and types of loss were confirmed by performance of otological examination and DPOAE and BERA. DPOAE confirmed absence of TOAE responses and BERA supported the conclusion of the diagnosis. The children who presented unilateral or bilateral sensorineural hearing loss were referred to hearing aid fitting and communication rehabilitation.
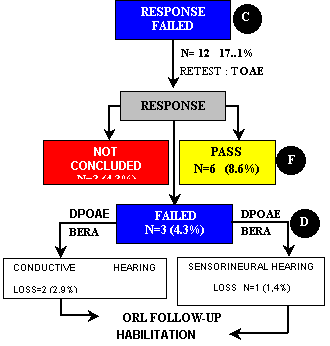
Of the 70 ears of group III, 12 (17.1%) did not pass the first step. TOAE were repeated and we observed that six ears (8.6%) passed, three (4.3%) failed and three (4.3%) were not concluded, for the same reasons described above. Of the three ears (4.3%) that did not pass TOAE again, two (2.9%) had the diagnosis of conductive hearing loss and one (1.4%) of sensorineural hearing loss (Figure 1, Flow Chart 2). Diagnosis was confirmed by otological examination by the otologist with DPOAE and BERA. The management of conductive and sensorineural cases was the same adopted for group II.
When we applied the statistical test to compare the results of the two groups on the second step, we observed that in the initial step (AxC) of "fail" responses, there was no statistically significant difference between groups II (n=29) and III (n=12), value of p=0.41. The same comparison between groups II and III was made for the next phase (ExF) of "passed" responses (14 and 6, respectively), and there was no statistically significant difference between them (p=0.48). In the initial stage (BxD) of "fail" responses, the comparison between groups II (11) and III (3) was not statistically different (p=0.38). We did not observe, therefore, differences between the groups of patients on the second step of the group concerning "pass" and "fail". We contrasted the proportions of conductive and sensorineural hearing loss. For group II (n=7 for conductive hearing loss and n=4 for sensorineural hearing loss), we did not observe statistically significant difference (p=0.10). For group III (n=2 for conductive hearing loss and n=1 for sensorineural hearing loss), we did not observe statistically significant difference (p=0.16) and upon comparing groups II and III, we did not observe statistically significant difference (n=9 of group II and n=5 of group III), p value = 0.066.
In the total examined population (groups I, II and III - 314 ears), we observed that 235 ears (74.8%) passed and 39 (12.4%) failed before hospital discharge, 20 ears passed (8.9%), two ears (0.7%) failed and 10 (3.2%) did not have a concluded diagnosis because they did not come back after hospital discharge. Out of 314 ears (100%), we observed that 283 ears passed TOAE; nine failed because of conductive hearing loss; five failed because of sensorineural loss, and 17 did not finish the procedures. In order to have an estimate of hearing loss and population size, the statistical analysis was made considering 95% confidence interval. In our sample, we observed that for conductive hearing losses the proportion was 29 ears to 1,000 and for sensorineural losses, it was 16 ears to 1,000. For the proportion to the general population, we observed an estimate of 1.04% to 4.76% for conductive losses, that is, variation of 10.4/1,000 to 47.6/1,000. For sensorineural hearing losses, the proportion was 0.2% and 2.99%, that is, between 2/1,000 and 29.9/1,000.
We have also checked the influence of incubator, use of ototoxic drug and mechanical ventilation in the three groups. As to incubator, we noticed that in the presence of this factor and "pass" response, the difference between groups I and II, I and III was statistically significant (p<0.0001) and between groups II and III it was not significant (p=0.083). For the "failed" response and use of incubator, we noticed statistically significant difference between groups I and III, II and III (P<0.001) and significant between groups I and II (p=0.0062). As to ototoxic medication, we noticed that for "pass" and this factor, there statistically significant differences for groups I and II, I and III (p<0.0001) and no statistically significant difference for groups II and III (p=0.123). For "failed" responses and ototoxic medication, we observed statistically significant differences for groups I and II (p=0.012) and II and III (p=0.16) and between groups I and III (p<0.0001). As to mechanical ventilation, we observed exposure to the factor and "pass" response with statistically significant difference for groups I and II, I and III (p<0.0001) and between groups II and III (p=0.0026). For "fail" response and use of mechanical ventilation, we observed that the difference was not statistically significant between groups I and II (p=0.1056) and it was significant between groups I and III, II and III (p<0.0001).
We analyzed the odds ratio of hearing loss, present and absent TOAE responses in the first step of the study, influenced by gestational age in the total population (314 ears) and separated by AIG pre-term group (group II) and PIG pre-term group (group III) to check the association of both variables.Figure 1.
Flow Chart 1 - Second stage of tests of newborns in group II (PT-AIG) that failed the first step, according to type and frequency of presented response and clinical management (N=158).
Note Percentages were calculated based on total number of ears in group II (N=158).
Flow Chart 2 - Second step of tests in newborns of group III (PT-PIG) that failed the first step, according to type and frequency of presented responses and clinical management (N=70).
Note Percentages were calculated based on total number of ears in group III (N=70).
Discussion
Data of the present study were similar to those reported by Smurzynski et al.16 who found a "pass" rate of 90% of term newborns; in our study, we found a "pass" rate of 95% - and 80% for pre-term babies - and 77% for AIG and 83% for PIG. The authors mentioned above did not separate children based on weight at birth and gestational age.
Namyslowski et al.17 observed a trend towards reduction of TOAE responses in low weight newborns, especially in pre-term babies. The same was detected in our study, since we observed statistically significant differences between groups I and II, I and III, II and III in "pass" rate. The difference between groups I and III was more significant, considering that it was the low birth weight group (PIG). Kok et al.18 assessed children with very low birth weight (610 to 1,590g) and, upon comparing their results to a previous study, they concluded that these children are more difficult to assess. Healthy newborns presented 97% of TOAE responses, whereas low birth weight babies had 71%. In our findings, the responses presented higher percentage in the PIG group (83%) when compared to this study, considering that weight variation was lower in children from the study by Kok et al.18, which may justify the occurrence of fewer TOAE responses.
Uziel & Piron19 assessed 110 ears of term babies and 80 ears of children at risk, including low birth weight. The percentage of "pass" for the healthy group was very similar to the results of our study for group I, without risk of hearing impairment (95%).
Zanten et al.20 observed present TOAE in low weight pre-term babies with mean weight of 1,040g, similar results to ours reported for group III. Salamy et al.21 assessed 224 children with gestational age of 24 to 34 weeks and low birth weight who remained hospitalized in the neonatal intensive care unit. Uziel & Piron19 observed that TOAE were more difficult to be recorded in newborns whose gestational age was below 35 weeks, justified by shape and size of the external auditory canal or immaturity of outer hair cells. These data were also observed in our study. Kok et al.18 performed TOAE in children with mean gestational age of 29 weeks and 7 days. The data were similar to the data reported by our study, since we had one 27 week and 4-day newborn with positive TOAE response, still in the neonatal period.
We observed higher chronological age at diagnostic conclusion in our study in groups II and III, comprising patients that required more care and management, which was a more vulnerable group to auditory pathologies and immaturity of the peripheral and central auditory system, when compared to group I.
In the study by Kanne et al.22 they recorded 11.6% of hearing loss in 5 children, a rate very similar to our findings, in which we observed 7% in group II and 4.3% in group III, amounting to 11.3% of the studied ears. White et al.23 found a prevalence of 5.95 for 1,000 for sensorineural hearing loss and 20 for 1,000 for conductive hearing loss. Watkin24 confirmed hearing loss in a proportion of 2/1,000; he recorded 19 cases of hearing loss that had failed the neonatal screening, in a proportion of 1.5/1,000 children with hearing loss. Stewart et al.25 observed a proportion of 2.7/1,000 for sensorineural hearing loss in newborns. Chapchap & Segre6 reported a proportion of 2.3/1,000 live babies for hearing impairment and diagnosed 2.2% of hearing impairment in the studied population.
We observed a higher percentage among ears that passed TOAE, both before and after the hospital discharge. These data are in agreement with many authors, who defended the importance of neonatal hearing screening and observed more failures than present responses10, 21, 15, 17, 18, 23, 24, 26, 27, 28.
As previously observed, conductive hearing loss is always more prevalent among children and newborns. These data are in accordance with the reports by White et al.23 ; Watkin24; Soares et al.26; Kanne et al.22; Vallejo et al.27; Cone-Wesson et al.28; Chapchap & Segre6.
It is important to highlight that intensive care has also been observed in our sample, especially for members of group III. We decided not to discuss this issue separately since the newborns who are in the neonatal intensive care unit are frequently managed in the incubator, use ototoxic medication and mechanical ventilation. We noticed that prematurity is a potential risk factor for hearing impairment, both for the general premature group and for the groups divided by weight at birth and gestational age. The likelihood of premature babies having hearing impairment is always higher than that of term babies.
Thornton et al.29 and Thornton30 observed that the middle ear pressure and external auditory canal obstruction are factors that influence pass and fail in OAE, a fact that was also observed in our study.
Conclusions
- The term newborn group presented higher percentage of present responses at TOAE when compared to the premature groups. Premature newborns failed more OAE than term children.
- The prevalence of conductive hearing loss in this population was of 29 ears for 1,000 and for sensorineural hearing loss it was 16 ears to 1,000. For the population of general newborns, the variation was from 10.4 to 1,000 to 47.6 to 1,000 for conductive hearing loss and from 2 to 1,000 to 29.9 to 1,000 for sensorineural hearing loss. Middle ear effusion cases were more frequent in the premature group.
- Low birth weight children were more difficult to be assessed and required longer follow-up.
- We observed pass rates in TOAE as from 27 weeks of gestational age.
- We did not find statistically significant difference between pass and fail rates for the groups of premature and small children for gestational age, for children who needed audiological assessment (second stage of the study).
- Incubator, ototoxic medication and mechanical ventilation were factors that influenced fail at OAE in the premature groups, especially the ones who were too small for the gestational age.
- The inspection of the external auditory canal or otoscopy is important to associate results and close the audiological diagnosis.
- Premature newborns are more likely to have compromise of OAE responses.
- We have to test all newborns and define protocols that reduce the number of lost cases and non-screened children so that we can effectively provide early diagnosis.
- EOAE technique is an important instrument for neonatal hearing screening.
- The combination of objective techniques of EOAE (TOAE and DPOAE) and BERA is necessary to come to a diagnosis about the auditory function and differential diagnosis.
- Early diagnosis of hearing loss is the role of a multidisciplinary team - neonatologist, pediatrician, otorhinolaryngologist, speech and hearing pathologist, nurse, family members - and it should be immediately followed by early intervention programs.
References
1. Northern JL, Downs MP. Audição em crianças. 3.ed. São Paulo: Manole; 1989.
2. American Speech Language Hearing Association (ASHA). Joint Committee on Infant Hearing: year 2000 Position Statement: Principles and Guidelines for early hearing detection and intervention programs. Available at: www.asha.org/infant_hearing/y2kpstn_stmnt.htm accessed in August 19, 2001.
3. Azevedo MF de. Avaliação e acompanhamento audiológico de neonatos de risco. Acta Awho set/dez 1991;10(3):107-116,.
4. Gomes CF, Crivari NMF. Os ruídos hospitalares e a audição do bebê. Rev Bras Otorrinolaringol 1998 set/out;64(5):453-457.
5. Costa SMB Da, Costa Filho OA. O estudo dos potenciais evocados acusticamente do tronco cerebral em recém-nascidos pré-termo. Rev Brasil Otorrinolaringol 1998 mai/jun;64(3):231-238.
6. Chapchap MJ, Segre CM. Universal newborn hearing screening and transient evoked otoacoustic emission: new concepts in Brazil. Scand Audiol (Suppl) 53:33-36, 2001.
7. Recomendação 01/99 do Comitê Brasileiro sobre perdas auditivas na infância. Jornal do Conselho Federal de Fonoaudiologia maio/jun 2000;5:3-7.
8. Oliveira JAA. Antibióticos e ototoxicidade. In: Sih T. (org). Infectologia em otorrinopediatria. Uso criterioso de antibióticos em infecções das vias aéreas superiores. Rio de Janeiro: Revinter; 2001. cap.39, p.223-230.
9. Yoshinaga-Itano C. Efficacy of early identification and early intervention. Seminars in hearing 1995 may;16(2):115-123.
10. Kemp DV & Ryan S. Otoacoustic emission tests in neonatal screening programmes. Acta Otolaryngol 1991 (Stockh) (Suppl) 482:73-84.
11. Eckley CA, Duprat A, Lopes Filho O. Revisão: emissões otoacústicas. Rev Bras Otorrinolaringol 1993;59(1):41-45.
12. Lopes Filho O, Carlos RC, Rossi HJZ, Eckley CA, Berezin A, Gallacci CB. Emissões espontâneas em recém-nascidos de risco. Rev Bras Otorrinolaringol 1997 nov/dez;63(6):567-573.
13. Probst R & Harris F. Otoacoustic emissions. Advances in Otorhinolaryngology 1997;53:182-204.
14. Norton SJ & Stover LJ. Emissões otoacústicas: um novo instrumento clínico. In: Katz J. Tratado de audiologia clínica. São Paulo: Manole; 1999. cap.29, p.444-458.
15. Norton SJ, Gorga MP, Widen JE, Folsom RC, Sininger YS, Cone-Wesson B, Vohr BR, Mascher K, Fletcher K. Identification of neonatal hearing impairment: evaluation of transient evoked otoacoustic emission, distortion product otoacoustic emission and auditory brain stem response test performance. Ear and Hearing 2000 oct;21(5):508-528.
16. Smurzynski J, Jung MD, Lafreniere D, Kim DO, Kamath MV, Rowe JC, Holman MC Leonard G. Distortion- product and click- evoked otoacoustic emissions of pre-term and full-term infants. Ear and Hearing 1993;14(4):258-274.
17. Namyslowski G, Morawski K, Urbaniec N, Lisowska G, Trybalska G, Bazowska G, Oslislo A. The hearing system in newborns from the Upper Silesia. Assessment of TEA depending on selected parameters of delivery disorders. Scand Audiol (Suppl) 2001;52:21-24.
18. Kok MR, Zanten GA Van, Brocaar MP, Jongejan HTM. Click-evoked otoacoustic emissions in very-low-birth-weight infants: a cross- sectional data analysis. Audiology 1994 may/jun;33(3):152-164.
19. Uziel A & Piron JP. Evoked otoacoustic emissions from normal newborns and babies admitted to an intensive care baby unit. Acta Otolaryngol (Stockh) (Suppl )1991;482:85-91.
20. Zanten BGA Van, Kok MR, Brocaar MP, Saver PJJ. The click-evoked otoacoustic emission, c-EOAE, in preterm-born infants in the post conceptional age range between 30 and 68 weeks. International Journal of Pediatric Otorhinolaryngology 1995 (Suppl);32:187-197.
21. Salamy A, Eldredge MA, Tooley WH. Neonatal status and hearing loss in high-risk infants. The Journal of pediatrics 1989 may;114:847-852.
22. Kanne TJ, Schaefer L, Perkins JA. Potential pitfalls of initiating a newborn hearing screening program. Arch Otolaryngology Head Neck Surg 1999 jan;125:28-32.
23. White KR, Vohr BR, Maxon AB, Behrens TR, Mcpherson MG, Mauk GW. Screening all newborns for hearing loss using transient evoked otoacoustic emissions. International Journal of Pediatric Otorhinolaryngology 1994;29:203-217.
24. Watkin PM. Outcomes of neonatal screening for hearing loss by otoacoustic emission. Archives of Disease in Childhood 1996 nov;75(3):158-168.
25. Stewart DL, Mehl A, Hall JM, Thomson, Carrol M, Hamlett J. Universal newborn hearing screening with automated auditory brainstem response: a multisite investigation. J Perinatol 2000 Dec;20(8 pt 2):128-131.
26. Soares E, Guerrero SMA, Azevedo MF de. Estudo comparativo das triagens auditivas por emissão otoacústica evocada transiente, observação comportamental e medidas de imitância acústica em crianças com e sem risco para deficiência auditiva. Rev Bras Otorrinolaringol 1998 mai/jun;64(3):221-227.
27. Vallejo JC, Oliveira JAA, Silva MN, Gonçales AS, Andrade MH. de. Análise das emissões otoacústicas em crianças com e sem risco auditivo. Rev Bras Otorrinolaringol 1999 jul/ag;65(4):332-336.
28. Cone-Wesson B, Vohr BR, Sininger YS, Widen JE, Folsom RC, Gorga MP, Norton SJ. Identification of neonatal hearing impairment: infants with hearing loss. Ear and Hearing 2000 oct;21(5):488-507.
29. Thornton ARD, Kimm L, Kennedy CR, Cafarelli-Dees D. External and middle ear factors affecting evoked otoacoustic emissions in neonates. British Journal of Audiology 1993;27(5):319-327.
30. Thornton ARD. Maturation of click evoked otoacoustic emissions in the first few days of life. Series in Audiology 1999;1:21-32.
[1] Speech and hearing pathologist, Master in Maternal-Children Program and Public Health, Nursing School, Ribeirão Preto, University of São Paulo (EERP/USP); Professor, Course of Speech and Language Pathology and Audiology, University of Franca.
[2] Professor, Assistant Physician, Department of Ophthalmology, Otorhinolaryngology and Head and Neck Surgery, Hospital das Clínicas, Medical School, University of São Paulo (FMRP-USP).
[3] Faculty Professor, Department of Ophthalmology, Otorhinolaryngology and Head and Neck Surgery, Hospital das Clínicas, Medical School, University of São Paulo (FMRP-USP).
Affiliation: Hospital das Clínicas, Medical School, University of São Paulo (FMRP-USP).
Address correspondence to: Cristiane Fregonesi Dutra Garcia - Rua Rui Barbosa, 267 Centro - 14680-000 - Jardinópolis - SP - Tel. (55 16) 3663-6885/ 3763-2981 - E-mail: crisfdg@megasinal.com.br
Study presented at 17º International Congress of Audiology, March 22 - 24, 2002, Bauru/SP.