Year: 2002 Vol. 68 Ed. 2 - (13º)
Artigo Original
Pages: 239 to 243
Auditory Evaluation (tone audiometry and evoked otoacustic emissions - distortion product) in patients with Systemic Lupus Erytematosus and Rheumatoid Arthritis
Author(s):
Súnia Ribeiro 1,
Chiara L. de O. Moraes 1,
Gabriele Righetti Neto 1,
José Alexandre Médicis da Silveira 2,
Fabiana Gonçalez 3,
Sandra Murad Quintero 4
Keywords: tonal audiometry, otoacustic emissions, systemic lupus erytematosus, rheumatoid arthritis
Abstract:
Aim: To evaluate the clinical and auditory behavior of the autoimmune disease patients, particularly Rheumatoid Arthritis (AR) and Systemic Lupus Erytematosus (LES) and to compare the audiometry findings and distortion product otoacoustic emissions in these patients. Material and Method: We selected 40 autoimmune disease patients, 27 classified as had AR and 13 (LES); 95% were female, average was 56years old in group of the AR and 29 years old in group of the LES. All patients underwent a thorough otolaryn-gological and clinical investigation, tonal audiometry and distortion product otoacoustic emissions. Results: We studied 80 ears, in 19 (19/54-35%) we observed alteration in the tonal audiometry in group of the AR and only in four (4/26-15,4%) in group of the LES. Talking about the otoemissions, we detected alterations in 37 ears (37/54-68,5%) from the AR group and in eight (8/26-30,7%) from the LES group. From the total amount of 80 ears, 23 (28,8%) presented alteration in the audiometry and 45% showed alteration in the otoacoustic emissions. Conclusion: The authors suggest that the distortion product otoacoustic emissions can be more sensitive than the tonal audiometry in the screening of cochlear alterations in the autoimmune diseases. We need a long-term follow-up to confirm the predictive value of the otoacoustic emissions on the otologic prognosis of these patients.
![]()
INTRODUCTION
The study of inner ear immune-mediated diseases is relatively new. It started about four decades ago, first in Germany and then in Japan, with the study by Lehnhart1 in 1958, about anti-cochlear antibodies, and by Kikuchi2, in 1959, about the sympathetic otitis. Beichert3, in 1961, observed autoimmune reactions in cochlear histologic sections.
As from the 70's, a period of better understanding of inner ear damage started. Sensorineural hearing loss that used to be seen with reserve because of poor treatment outcomes became better understood thanks to the conduction of a number of studies about the involved etiopathogenic mechanisms4, 5, 6.
McCabe7, in 1979, reported 17 cases of sensorineural hearing loss that presented good response to corticosteroid treatment. In the same study, the author noticed the presence of alternated results to the lymphocytic migration test, assuming the participation of immunologic mechanisms and, for the first in the literature, the diagnosis of autoimmune hearing loss was made.
The knowledge about autoimmune diseases and their correlation with the clinical picture of sensorineural hearing loss (SNHL) contributed significantly to the understanding of this type of hearing loss, enabling the performance of specific treatment approaches.
Autoimmune diseases (AID) may be classified into organ-specific diseases (primary) - antibodies directed to antigens of only one organ of the body, such as Hashimoto thyroid, and non organ-specific diseases (secondary) - antibodies directed to antigens of different tissues, leading to systemic disease, such as systemic lupus erythematosus (SLE).
The inner ear may be included into the group of organ-specific diseases, or more commonly, the organ compromised by the systemic form, resulting in hearing loss as the first symptom. The rate of inner ear impairment in systemic autoimmune hearing loss is variable. Doig et al.6 observed SNHL in nearly 50% of the patients with rheumatoid arthritis (RA). Other authors correlated immunologic mechanisms in the pathogenesis of various forms of auditory compromise, such as fluctuating loss, rapidly progressive sensorineural loss, sudden deafness and even some types of Ménière disease8,9.
SNHL involving immunologic mechanism is more commonly found in female subjects, between the 3rd and 5th decades of life, presenting sudden onset and progression to moderate or severe hearing loss in few months.
In the series by McCabe10, the age of patients varied from 19 to 42 years, with no gender differences. Hughes et al11 reported that hearing loss may start at any age, being more common in middle aged women.
Audiometry curves of autoimmune SNHL do not have a characteristic pattern and they may be either ascending or descending. Vocal discrimination may be normal or abnormal, regardless of the pure tone curve results.
Recording of evoked otoacoustic emissions (OAE) is the newest method to detect cochlear auditory losses. OAE were first described by David Kemp12 in 1978, who defined them as a release of sound energy in the cochlea that propagates to the middle ear up to the external auditory canal.
OAE may be classified in two categories: spontaneous and evoked13. Evoked otoacoustic emissions are divided into 3 types, according to the nature of the stimulus: transient, stimulus-frequency and distortion product. Distortion product, which was used in our study, is obtained by a click stimulus that consists of two different pure tone frequencies (f1 and f2). The response is characterized by a third sound whose frequency is usually the distortion product of 2f1-f2, whose ratio is approximately 1.214, 15.
Distortion product is a type of emission that is like an immediate response to an acoustic stimulus and it may be generated by any frequency, by means of selected stimuli, evaluating cochlear activity in specific frequencies. Owing to its similarity with the audiometry curve and the possibility of assessing the hair cell functions in different sites of the cochlea, distortion product otoacoustic emissions provide wide clinical application when compared to transient evoked otoacoustic emissions. Distortion product otoacoustic emissions may be obtained in the frequencies 0.75 and 8kHz and in subjects with thresholds of up to 50dB HL16.
The purpose of the present study was to assess clinical and auditory behavior of patients with AID, especially those with RA and SLE and to compare the symptoms with audiometric findings and distortion product otoacoustic emissions of these patients.
material and method
We selected 40 patients with established clinical picture of autoimmune disease (rheumatoid arthritis and systemic lupus erythematosus) followed in the ambulatory of the Department of Rheumatology of Hospital Santa Marcelina, São Paulo, between March 2000 and March 2001.
The criteria for exclusion from the study were: contact with loud sounds, middle ear affections, family history of hearing loss, previous use of aminoglycosides and/or loop diuretics, and syphilis.
Among the 40 selected patients, 38 (95%) were females and 2 (5%) were males, being 27 (67.5%) with RA and 13 (32.5%) with SLE.
Ages ranged from 16 to 71 years (median of 56 years) in the RA group and 16 to 40 years (median 29 years) in the SLE group.
Patients were questioned about tinnitus, vertigo/dizziness and ear fullness, and they were submitted to ENT and otoneurologic tests, including Fukuda, Romberg, sensitized Romberg tests, presence of spontaneous and semi-spontaneous nystagmus, cerebellar tests and assessment of cranial nerves. They were also submitted to audiometry and impedanciometry, distortion product otoacoustic emissions and Lues serology. Auditory tests were performed at Clínica Otorhinus, in São Paulo. Pure tone audiometry was performed in a sound-proof booth, using audiometer MAICO-MA 41 and acoustic immitance with impedanciometer Rexton Impedance Audiometer 128. As to distortion product otoacoustic emissions, we used the software Scout Sport (Distortion Product Otoacoustic Emissions Measurement System - program version 1.54) by Bio-login Systems Corporation, studying frequencies 0.75 to 8KHz.
We also studied the groups of rheumatoid arthritis and systemic lupus erythematosus separated, correlating presence of symptoms and audiometric findings and distortion product otoacoustic emissions using test x2 for p<0.005. The same analysis was used to assess the two groups as a whole.
RESULTS
Among the 80 studied ears, the most frequent complaint was tinnitus, reported in 30 ears (37.5%), followed by ear fullness in 9 ears (11.25%). Among the ears with tinnitus, 20 (66.7%) were in the AR group and 10 (33.3%) were in the SLE group.
Vertigo/dizziness was reported by 14 patients (35%), nine (64.3%) were in the AR group and 5 (35.7%) were in the SLE group.
Out of 40 studied patients, we did not observe abnormal results in the cranial nerve test nor in the balance test, cerebelllar test and nystagmus investigation.
Owing to lack of consensus to classify hearing loss using only isolated frequencies, the authors decided to analyze the pure tone threshold of each frequency, considering normal hearing for thresholds within -10 to 25dBHL.
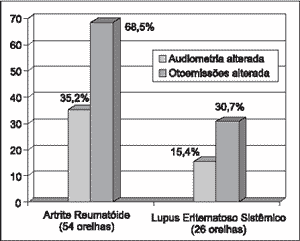
We detected abnormalities in the pure tone audiometry of 19 ears (19/54 - 35%) in the RA group and only 4 (4/26 - 15.4%) in the SLE group. Of the 80 ears with AID (RA and SLE) 23 (28.8%) presented abnormal audiometry. (Graph 1).
Of the 80 studied ears, sensorineural hearing loss in high frequencies (4, 6 and/or 8kHz) was the most prevalent (22/23-95.6%) at pure tone audiometry, the same noticed for otoacoustic emissions (37/45-82.2%).
As to otoacoustic emissions, 37 ears (68.5%) of RA group presented abnormalities, and among them 18 (48.6%) had normal audiometry. In the SLE group, in 8 ears (30.7%) we detected abnormalities and among them, 4 (50%) presented normal audiometry. Of the 80 ears with AID (RA and SLE), 45 (56.3%) presented abnormal otoacoustic emissions (Graph 1).
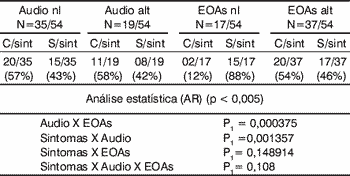
In the group of rheumatoid arthritis, there was statistical significance in the correlation of findings of audiometry and OAE and in presence of symptoms and audiometry abnormalities. There was no significant correlation between symptoms and audiometric findings and otoacoustic emissions (Table 1).
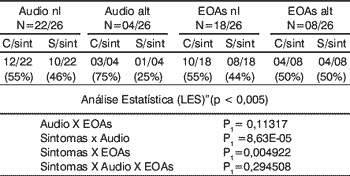
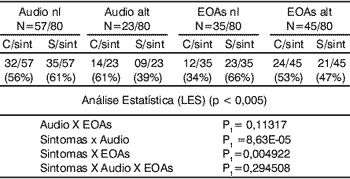
In the group of systemic lupus erythematosus there was statistically significant difference in the correlation of findings of audiometry and presence symptoms, and in the presence of symptoms and abnormal otoacoustic emissions. There was no statistically significant correlation between audiometry findings and otoacoustic emissions, nor between symptoms and audiometry findings and otoacoustic emissions (Table 2). Upon studying both groups together, there was statistically significant difference in the correlation of audiometry findings and otoacoustic emissions, and presence of symptoms and audiometry findings. There was no statistically significant difference between presence of symptoms and otoacoustic emissions abnormalities, nor when we compared symptoms and audiometry and otoacoustic emission findings (Table 3).
Graph 1. Distribution of audiological findings (n = 80 ears).
Table 1.
Table 2.
Table 3.
DISCUSSION
Since the publications by McCabe7,10, suggesting by the first time the clinical correlation between SNHL and AID, we could observe, as from 1979, a greater number of publications advocating that autoimmune cause was the main diagnostic hypothesis for many cases of SNHL without apparent cause9, 11, 17.
The key part of sensorineural autoimmune diagnosis is clinical history, since there are no definite laboratory tests yet16. The histopathology confirmation is not possible, since the inner ear is inaccessible for tissue biopsy. Bowman and Nelson18 showed that the association of autoimmune SNHL and HLA-CW7 may be a diagnostic marker that will facilitate the selection of patients to treatment.
SNHL may initially manifest as an isolated symptom but it is more commonly found as part of a immune-mediated systemic disease9, 11, 19.
The present study, according to the study by Hughes10, has shown that SNHL involving the immune mechanism may be started at any age, more commonly in women.
Samara et al20 studied 18 patients with RA grades I to IV concerning results of pure tone audiometry and impedanciometry. They concluded that although they had not been able to define a correlation between intensity of joint manifestations and inner ear dysfunction, the occurrence of latent hearing loss (pure tone audiometry and recruitment) and sensorineural hearing loss (high frequency loss and recruitment) in 66.6% of the patients, not correlated to age range and co-morbidities, implies a form of rheumatoid aggression to cochlear structures. In the present study, among the 54 ears of the RA group, 19 ears (35%) showed abnormal pure tone audiometry and 37 ears (68.5%) presented abnormal otoacoustic emissions, among them, 18 (48.6%) had normal audiometry. In the present study, there was statistic significance in the correlation of findings of audiometry and otoacoustic emissions in the group of rheumatoid arthritis, as well as in the presence of symptoms and audiometry abnormalities. There was no statistically significant difference between symptoms and otoacoustic emissions, nor when we compared symptoms and audiometry findings and otoacoustic emissions in this group.
Atra et al21 performed pure tone audiometry, vocal discrimination and impedanciometry in 30 patients with SLE and compared them to the control group. The results were normal in the two groups and they concluded that SLE and drug treatment of the underlying disease do not produce hearing abnormalities. Different from these findings, our results showed that patients with SLE presented abnormal audiometry in 4 ears (4/26 - 15.4%), and in eight of them (30.70%) there was alterations of otoacoustic emissions (distortion product).
Of the 80 ears with AID (RA and SLE), 23 (28.8%) presented abnormal audiometry and 45 (56.3%) had otoacoustic emission abnormalities. Sensorineural hearing loss in high frequencies (4, 6 and/or 8kHz) was more prevalent on pure tone audiometry (22/23 - 95.6%) and otoacoustic emissions (37/45 - 82.2%).
When we compared the different groups and the presence of symptoms with audiometry findings and otoacoustic emissions abnormalities, the authors observed that there was statistic significance in the correlation of audiometry findings and otoacoustic emissions, and in the presence of symptoms and audiometry abnormalities in the group of rheumatoid arthritis. There was no significant correlation between symptoms and otoacoustic emissions, nor between symptoms and audiometry and otoacoustic emissions findings. In the group of systemic lupus erythematosus, there was statistically significant difference in the correlation of audiometry findings and presence of symptoms, and in the presence of symptoms and otoacoustic emissions abnormalities. There was no statistically significant difference between audiometry and otoacoustic emissions findings, nor between symptoms and audiometry and otoacoustic emissions findings. Upon studying the two groups together, there was statistically significant difference in the correlation of audiometry and otoacoustic emissions findings, and in presence of symptoms and audiometry abnormalities. There was no significant correlation between presence of symptoms and otoacoustic emissions abnormalities, nor between the comparison of symptoms and audiometric and otoacoustic emissions findings.
A possible explanation for lack of statistical correlation of presence of symptoms and audiometry and otoacoustic emissions, both in the isolated groups and in the 80-ear group as whole, would be the occurrence of biochemical or physiological alterations without anatomic damage9.
According to the results described above, the authors suggested that otoacoustic emissions may be a more sensitive test than pure tone audiometry to detect cochlear alterations of autoimmune diseases. To that end, a larger sample has to be studied and those with abnormal otoacoustic emissions have to be followed up concerning the evolution of the sensorineural loss, confirming the predictive value of the test for the audiological prognosis of the patients with AID.
CONCLUSION
The purpose of the present study was to investigate patients with autoimmune disease (specifically SLE and RA) and compare symptoms (vertigo/dizziness, ear fullness and tinnitus) with audiometry findings and otoacoustic emissions. Since audiometry and otoacoustic emissions abnormalities were most frequently found in high frequencies (4, 6, and 8kHZ), the authors emphasized the importance of using otoacoustic emissions to assess patients with AID.
The results of the present study suggested that distortion product otoacoustic emissions were more sensitive than pure tone audiometry to detect cochlear affections of autoimmune diseases but the study has to be continued in order to confirm the predictive value of otoacoustic emissions in the audiological prognosis of patients with AID.
The authors also highlighted the importance of counseling patients with AID about the possibility of having inner ear affection during the evolution of the underlying disease, proposing periodical audiological assessments in this group of patients.
REFERENCES
1. Lehnhart E. Plötzliche Hörstörungen auf briden Seiten gleichzeitig oder nacheinander aujgetreren. Z Laryngol Rhinol Oto 1958;37:1-17.
2. Kikuchi M. On the "sympathetic otitis". Zibi Rinsyo Kyoto 1959;52:600.
3. Beichert P. Zur Frage der Empfindungs Schwerhorigkeit und Autoallergie. Z Laryngol Rhinol Otol 1961;40:837.
4. Arnold W, Pjaltz R, Altermatt HF. Evidence of serum antibodies against inner ear tissues in the blood of patients with certain sensorineural hearing disorders. Acta Otolaryngol 1985; 99:4-37.
5. Schiff M, Yoo TJ. Immunologic aspects of otologic disease: an overview. Laryngoscope 1985;95:259.
6. Stephens SDG, Luxon L, Hinchcliffe R. Immunological disorders and auditory lesions. Audiology 1982;21:128-148.
7. Mccabe BF. Autoimmune sensorineural hearing loss. Ann Otol 1979;88:585-589.
8. Veldman JE, Roord JJ, O'Connor AF et al. Autoimmunity and inner ear disorders and immune complex mediated sensorineural hearing loss. Laryngoscope 1984;94:501.
9. Veldman JE. Immune-mediated inner ear disorders. New syndromes and then etiopathogenesis. In: Veldman JE & Mccabe BF. Oto-immunology. Amsterdam: Kugler Publ.; 1987. p.125-142.
10. Mccabe BF. Autoimmune inner ear disease: clinical varieties of presentation. in: Veldman JE & Mccabe BF. Oto-immunology. Amsterdan: Kugler Pube; 1987. p.143-148.
11. Hughes GB, Kinney SE, Barna BP, Hamid MA, Calabrese LH. Autoimmune Vestibular dysfunction: preliminary report. Laryngoscope 1985;95:893-897.
12. Kemp P, Alexander L, Brown AM. Acoustic Emission cochleography: Practical aspects. Scand Audiol Suppl 1986;25:71-95.
13. Probst R, Lonsbury - Martin BL, Martini GK. A review of otoacoustic Emissions. J Acoust Soc Am 1991;89(5):2027-2062.
14. Martin GK, Whitehead ML, Lonsbrery Martin BL. Potential of evoked otoacoustic emissions for infant hearing screening. Sem Hear 1990;11:186-204.
15. Norton SJ & Stover IJ. Otoacoustic Emission. In: Katz J (ed.). Handbook of clinical Audiology. 4th. ed. Baltimore: Williams & Wilkins; 1994. p. 448-462.
16. Ferreira LA, Silveira JAM, Percebo DD, Gonçales F. Perda Auditiva Induzida por Ruído: Análise dos Achados de Audiometria Tonal, Potenciais Evocados do Tronco Cerebral e Emissões Otoacústicas Evocadas por Produto de Distorção. Revista Brasileira de Otorrinolaringologia 2001;67(1): 9-14.
17. Harris JP. Autoimmune or immune-mediated causes of deafness. West J Med 1991;154:719-20.
18. Bowman CA & Nelson RA. Human leukocytic antigens in autoimmune sensorineural hearing loss; 1987:7-9.
19. Kanzaki J & O-Uchi T. Circulating immune complexes in steroid-responsive sensorineural hearing loss and the long-term observation. Acta Otolaryngol 1983 (Suppl.) 393:77-84, D.T.; Bray.
20. Samara AD, Marques-Neto JF, Ermel MH, Maudonnet O. Audição na artrite reumatóide: recursos da impedanciometria e audiometria tonal. Rev Bras Reumat 1975;2:86-90.
21. Atra E, Pontes PAL, Goldenberg J, Nóbrega JL, Sato EI, Pollar DF. Estudo da audição no lúpus eritematoso disseminado. Rev Bras Reumat 1983;23(5):203-207.
1 Resident Physician, Service of Otorhinolaryngology, Hospital Santa Marcelina.
2 Head of the Service of Otorhinolaryngology, Hospital Santa Marcelina, Director of Clínica Otorhinus, Ph.D., Assistant of Clinical Otorhinolaryngology, Hospital das Clínicas, Medical School, University of São Paulo.
3 Preceptor of resident physicians, Clínica Otorhinus and Hospital Santa Marcelina.
4 Audiologist, Clínica Otorhinus.
Study conducted at Hospital Santa Marcelina and Clínica Otorhinus (Centro de Estudos Alexandre Médicis da Silveira), São Paulo/SP.
Address correspondence to: Súnia Ribeiro - Rua Luís Góes,1328/6 - São Paulo/SP - CEP 04043-150 - Tel:(55 11) 5581.7464
Article submitted on December 3, 2001. Article accepted on February 7, 2002.