Year: 2002 Vol. 68 Ed. 2 - (2º)
Artigo Original
Pages: 157 to 165
Histomorphometryc aspects of the superior laryngeal nerve
Author(s):
Romualdo Suzano Louzeiro Tiago 1,
Mário Sérgio Lei Munhoz 2,
Flávio Paulo de Faria 3,
Arnaldo Guilherme 4
Keywords: larynx, superior laryngeal nerve, dysphagia, voice disorders.
Abstract:
Introduction: The larynx plays a primal role in the maintenance of life. The perfect integration between its afferent and efferent pathways, provided by the superior laryngeal nerve (SLN) and by recurrent laryngeal nerve, avoids foreign body aspiration and maintains the intrinsic laryngeal muscle tone by means of the reflexes elicited by the mucus wave movements. Aspirations and pneumonias as well as dysphonias, more frequently found among the elderly, might be triggered by disorders affecting these same neural pathways. Aim: The aim of this study was to assess the histomorphometryc characteristics of the SLN in elderly subjects in comparison to adults. Materials and method: A fragment of each side of the superior laryngeal nerve was obtained during necropsy examination and two different age groups were gathered. One group was made up of 5 adult subjects averaging 39,6 years of age and one was constituted of 6 elderly subjects averaging 71,7 years of age. The material was examined in a 40x objective lens microscope, connected to a computerized image analyzing system and the nerve's main parameters as the myelinated fibers frequency, fiber density per mm2, and intraperineural area were found. Results: A statistically significant reduction of the smaller myelinated fibers (1 and 2 mm) was observed in the Elderly group. No difference was noted in the myelinated fiber frequency of the left/right sides. Conclusion: Being the low diameter superior laryngeal nerve myelinated fibers functionally responsible for the reflex of glotic closure, their reduction in the elderly subjects may be related, alongside with other factors, to a greater predisposition to aspiration and voice disorders.
![]()
INTRODUCTION
The larynx performs essential functions in maintenance of life. Its most relevant functions in order of importance are protection of airways, breathing and phonation. The larynx protects the airways during swallowing, coordinates and optimizes the upper airways during breathing and promotes control of phonation by means of intrinsic mechanisms, together with the pharynx and oral and nasal cavities1,2.
A perfect integration between its afferent (sensorial) and efferent (motor) pathways is necessary for maintenance of its functions. The internal branch of the superior laryngeal nerve (SLN) is responsible for sensitivity of the supraglottic region up to the superior face of the vocal folds1,2,3 and the external branch, together with the recurrent laryngeal nerve (RLN), is also responsible for sensitivity of the subglottis4,5. The myelin and amyelin terminations of these nerves form a submucosal plexus and are in charge of the two main types of laryngeal sensitivity - mechanosensitivity and chemosensitivity3. The afferent impulses of the deep muscle receptors and cricothyroid joints, together with the submucosal plexus fibers, make up the internal branch of the SLN2.
The SLN, through its external branch, is responsible for the efferent innervation of the cricothyroid muscle, which pushes and moves the vocal folds close, by means of a communicating branch between the external branch and RLN. It is also partly responsible for the motor innervation of the thyroarytenoid muscle4,5. The other intrinsic laryngeal muscles are innervated by efferent myelin fibers that run through RLN. The interarytenoid muscle is likely to have a mixed efferent innervation, beginning in the internal branch of the SLN and RLN4,5.
Protection of the airways through the glottic closure reflex is a basic and involuntary function that depends on good operation of the laryngeal afferent and efferent pathways, and prevents aspiration during swallowing6. The elderly are more likely to have decreased airway protection reflex; consequently, they are more prone to aspiration of foreign bodies and pneumonias7,8.
The respiratory function of the larynx was better described by Negus (1949), who observed that the glottis opens in fractions of seconds before air passes, triggered by the diaphragm lowering. Phonation is the most complex, and probably the least understood, of the three functions, although it is the most frequently studied. Phonation results from production of a fundamental tone that is modified by resonance cavities corresponding to the upper aero-digestive tract. Apart from motor regulation, another mechanism to regulate the fundamental frequency is related to laryngeal sensorial information (mucosa, joint receptors and muscle afferent terminations). Through sensorial information in the supraglottis and subglottis, trained singers are able to position the larynx to achieve the desired fundamental frequency before emission of an audible sound2,9. Sensitivity derived from subglottis and supraglottis is responsible for triggering this reflex.
Voice alterations are very frequent in the elderly. According to Hirano, Kurita, Sakaguchi (1989)10 and Sato & Hirano (1997)11, presbyphonia could be related to alterations in vibration of the vocal fold mucosa and muscular atrophy.
Bearing in mind the importance of SLN and its branches, surgeons try to identify and preserve them in neck surgical procedures. Injury of the SLN external branch during thyroidectomy results in reduced vocal fold tension, secondary to cricothyroid muscle denervation, and inability to achieve higher frequencies12,13. In cases of partial supraglottic and supracricoid laryngectomy, care should be taken to spare the internal branch, thus maintaining sensitivity of arytenoid and pyriform sinus mucosa, making patient rehabilitation easier and decreasing the risk of aspiration and dysphagia in the postoperative period14.
Taking into account the importance of the superior laryngeal nerve as to its functions of airway protection and control of the phonatory mechanism, we decided to carry out this study based on the fact that in our clinical practice the elderly patients usually complain of dysphonia, choking and dysphagia, and not always present alterations in the examinations available.
The objective of this study is to assess the histomorphometric characteristics of the superior laryngeal nerve in elderly individuals and to compare the findings with adults in order to identify morphological parameters. These parameters could provide subsidies justifying the most frequently observed alterations in the elderly, with decreased laryngeal sensitivity and voice alterations.
MATERIAL AND METHOD
The research project was assessed and approved by the Research Ethics Committee of São Paulo Hospital- UNIFESP and by the Ethics Committee for Research Project Analysis (CAPPesq) of the Medical School Training Hospital - USP, in order to make specimen collection in the Death Verification Service of the City of São Paulo (SVOC), University of São Paulo, feasible.
Fragments of right and left SLN, one-centimeter long, of 11 corpses submitted to necropsy at the Death Verification Service of the City of São Paulo, USP, were collected from November 2000 to March 2001. The fragments were collected between 7 hours and 30 minutes and 19 hours and 30 minutes after death, and were kept in a solution for posterior analysis at the Electron Microscopy Center (CEME), UNIFESP/EPM.
The corpses selected were male, Caucasian, with no past history of diabetes, alcoholism, malignant neoplasm and sudden weight loss15. The corpses were excluded from the study taking into account the past history based on information given by relatives or included in the death record. Two groups were assembled: 1) Adult group, with individuals aged 40 or under, composed by five corpses, and 2) Elderly group, with individuals aged over 60, composed by six corpses.
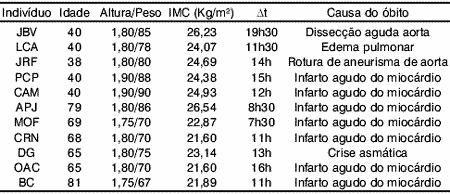
Table 1 shows the initials, age, weight, height, body mass index, time from death up to collection of fragment and cause of death.
Material collection
The material was collected by cervical dissection starting at the arciform incision, usually performed in necropsy, from one shoulder to the other and passing below the sternal furcula. The dissection was bilaterally performed at a plan below the platysma up to the mandible angle.
After identifying the anterior margin of sternocleidomastoid muscle, we made a deeper dissection towards the major cervical vessels trying to find the superior laryngeal nervous-vascular bundle and the internal and external branches of SLN; the nerve fragment was collected 3 cm away from the thyrohyoid membrane7. The dissection was performed on the right and left sides of each corpse.
The fragments were immediately kept in separate and labeled recipients with modified Karnovsky solution16, containing glutaraldehyde 2.5%, paraformaldehyde 2% in sodium cacodylate 0.1 M buffer, pH 7.4, at room temperature, for a minimum period of two hours. During collection the fragments were cross-sectioned (perpendicular to the nerve length axis) in order to obtain the following histomorphometric data: total cross-section area of the nerve (including epineuro and perineuro), intraperineural area (area representing the number of myelin fibers), number of myelin fibers and classification of fibers according to their diameter.
Preparing tissue for light microscopy
Specimen preparation and morphometric evaluation were carried out at CEME-UNIFESP/EPM. After initial fixation with modified Karnovsky solution (1965), the fragments were submitted to the following steps:
1. Four successive lavages in sodium cacodylate 0.1 M buffer, pH 7.4;
2. Post-fixation with osmium tetroxyde 2% in sodium cacodylate 0.1 M buffer, pH 7.4;
3. Infusion in aqueous uranyl acetate solution 0.5% for 30 minutes, followed by two quick lavages with distilled water.
4. Dehydration at the following increasing ethanol concentrations: 70%, 90% and 100%. Afterwards the material was twice infused in propylene oxide.
5. Infiltration in a resin mixture similar to Araldite 502â: propylene oxide, at 1:3, 1:2, 1:1 concentrations. After this stage the material was kept in pure resin. Afterwards the tissue fragments were transferred to inclusion moulds and horizontally oriented to enable cross-section. Then they were taken to an oven at 60o C, for 72 hours, for resin polymerization.
The blocks obtained after this preparation were cut using an ultramicrotome LeicaT, model Reichert Ultracuts, with glass blades, in order to have 0.3-µm-thick semi-thin sections. The sections were later placed in slides and dyed with toluidine blue at 1%.
Morphometric Study
The sections were evaluated using a light microscope NikonT, model Optiphot II, with 10x and 40x lens and final magnification of 215x and 860x, coupled to an image analysis system LeicaT, model Quantimet 500 QWIN. In this system, the images of the field evaluated were digitized through a high-resolution video camera SonyT, model XC 003P and transferred to a computer.
Morphometric evaluation by light microscopy was divided into two stages, as follows:
- Obtaining measurement of total cross-section area and SLN intraperineural area. In order to obtain the area measurements, the nerve images were digitized using a 10x lens, representing a final magnification of 215x in the computer screen. The values of the total and intraperineural areas were obtained by means of software. Obtaining the intraperineural area was important to calculate the myelin fiber density per mm2 and the total number of fibers of the nerve based on a representative sample.
- The number and the external diameter of SLN myelin fibers were calculated. The final magnification of the image on the screen using 40x lens was 860x. This image was digitally magnified twice to get the external diameter of myelin fibers, providing a final magnification of 1,720x. Approximately two or three fields were randomly evaluated in each slide. Therefore, the area evaluated ranged from 6.13% to 15.3%, depending on the slide. Based on the intraperineural area obtained in the first stage and on the total area of the fields evaluated, the SLN myelin fiber total number and numerical density per mm2 were estimated.
To avoid sampling error (margin effect), the myelin fibers projected on the lower and left lines delimitating the field were excluded17. The shortest fiber diameter (the longest distance perpendicular to the longest myelin fiber) was chosen to measure myelin fibers with an elliptical or irregular perimeter7,18.
Statistical analysis
The variable distributions were represented by frequency, mean and standard deviation. The Student's t test was used to compare ages and body mass index (BMI). The statistical method Repeated Measure ANOVA was used to compare intraperineural area and myelin fiber density and frequency means of the Adult and Elderly groups. The significance level adopted was 0.05. The p value (likelihood of an event) <0.05 was considered significant, leading to null hypothesis of equality between the groups.
The statistical analyses were carried out by the Biostatistics Discipline, Department of Preventive Medicine, UNIFESP-EPM, using the statistics SAS v. 6.12 software (SAS/STAT Software, 1997: Release 6,12 Cary, NC: SAS Institute Inc.).
RESULTS
By the Student's t test with unequal variances, we observe, in Table 2, that there is statistically significant difference in the mean age of the two groups (p<0.001). The Elderly group is, in average, 31.57 ±2.91 years older than the Adult group.
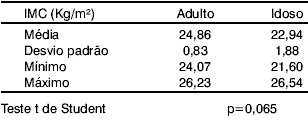
Table 3 shows, according to Student's t test, that body mass index mean of the Adult group is equal to that of the Elderly group (p=0.065).
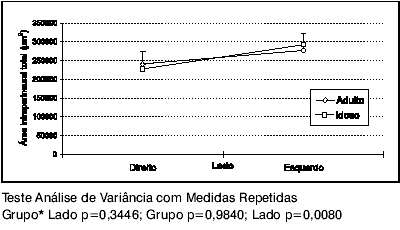
Comparing the intraperineural areas of the Adult and Elderly groups by the statistical model Repeated Measure ANOVA, chart 1 shows there is no statistically significant difference between the groups (p=0.9840). However, there is statistically significant difference between the mean right and left side areas (p=0.0080). The left side area is, on average, 51,215. 9 ±14,682.5 µm2, or 0.0512 ±0.0147 mm2 larger than the right side area.
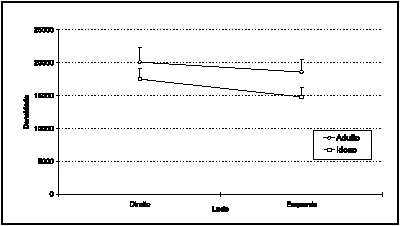
Using the statistical model Repeated Measure ANOVA to compare numerical densities of the Adult and Elderly groups, chart 2 shows there is no statistically significant difference between the groups (p=0.2042). Nevertheless, there is statistically significant difference between mean numerical density on the right and left sides (p=0. 0439). The right side has, on average, 2,158.2 ± 369.9 myelin fibers/mm2 more than the left side.
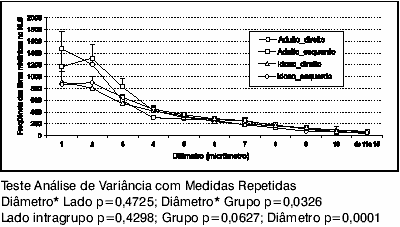
Chart 3 displays a unimodal curve in both groups, with greater amount of short diameter myelin fibers; in that, 1-µm myelin fiber are more common on the right, and 2-µm fibers, on the left, with no intragroup statistically significant difference (p=0.4298). The frequency distribution of myelin fibers according to their several diameters (p=0.0001) is statistically significant in both groups, with a predominance of shorter diameter fibers. There is no statistically significant difference between the two sides in both groups when comparing the frequency distribution of myelin fibers according to their several diameters (p=0.4725). Comparing the groups separately, there is no statistically significant difference, but there is a tendency to some difference (p=0.0627).
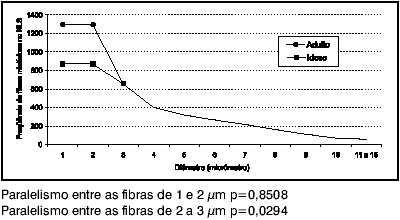
The shorter diameter myelin fiber (1 µm and 2 µm) are more frequent in the Adult group (p=0.0001), and, on average, there are 429 ±86 more myelin fibers in the Adult group than in the Elderly group (Chart 4). Considering 1- and 2-µm diameter, there is some similarity, or parallelism, in the frequency distribution behavior of myelin fibers (p=0.8508). As to 2- and 3-µm diameter, there is no parallelism in frequency distribution behavior of myelin fibers (p=0.0294); and over 3 µm, the frequency distribution becomes similar in both groups (p=0.7730).
Table 1.
Table 2.
Table 3.
Graph 1. Mean SLN intraperineural area profiles of the Adult and Elderly groups, according to right or left side.
Graph 2. Mean SLN numerical density profiles of the Adult and Elderly groups, according to right or left side.
Graph 3. Mean frequency of SLN myelin fiber profiles of the Adult and Elderly groups, right or left sides.
Graph 4. Distribution parallelism between SLN myelin fibers of the Adult and Elderly groups.
DISCUSSION
The aging process leads to changes in the body that are responsible for several types of manifestations depending on the organ under evaluation. We observed that the elderly somehow tend to present more voice and swallowing disorders, and these alterations are associated to some aspiration and, consequently, pneumonias.
Several studies have addressed the voice of the elderly, mainly regarding alterations in vocal fold movements and in structure of some laryngeal areas10,11. However, there are few studies assessing the laryngeal innervation taking into account the microscopic aspect and correlating the findings with different age groups.
The few studies on SLN and RLN histomorphometric evaluation do not achieve standardization of the material collection method, neither of the assessment method itself (fiber count, diameter and area measurement).
Some authors carried out investigations on myelin fiber frequency in normal individuals and animals and demonstrated different results. This fact could be related to the study of different species and lack of standardization on the nerve section point, a piece of information not always reported in the research studies published19.
The studies published so far about SLN histomorphometry in humans comprise a reduced number of individuals and there is no standardization on material collection and preparation methods, hindering comparison of results7,20.21.
Aiming at evaluating the SLN based on its histomorphometric characteristics, we selected a sample of individuals who had no past history of conditions that could interfere with the normal standard for their age group. Thus, observing Table 1 and the groups of individuals who made up our sample, the main cause of death in both groups was acute myocardium infarction, followed by rupture of aneurysm (more often in younger individuals), cardiogenic pulmonary edema and asthmatic exacerbation. All these were considered natural cause of death. When composing our sample, we tried to make two groups with very distinct age groups.
At first our purpose was to have a young Adult group, ages ranging from 20 to 30 years old, which would make more evident the possible differences with the Elderly group, aged over 60 years. Since the main cause of death in the 20-30 year age group is violent death, we had no access to these corpses, who are referred to the Legal Medicine Institute of São Paulo. Our permission for collection was restricted to the Death Verification Service of the City of São Paulo (SVOC-USP), where necropsies are performed in individuals with a natural cause of death. This fact made the study with a younger group unfeasible. The mean age of our groups was 39.60 years for Adults and 71.17 years for Elderly, unlike the groups studied by Mortelliti et al (1990), who reported mean age of 23.8 years for Adults and 76.2 years for Elderly. The main cause of death of the Young group in their study was multiple traumas.
We excluded from our sample the individuals with conditions associated to development of peripheral polyneuropathy, such as diabetes, alcoholism, neoplasm, sudden weight loss and use of chemotherapy, as suggested by Dyck (1989).
We could not find in the literature any study that has evaluated the SLN on both sides in individuals at different ages. Mortelliti et al (1990) assessed the nerve only on the right side. Taking into account that the main function of SLN is supraglottis and part of subglottis sensitivity1,2,3,4,5, and that there is no difference in sensitivity between the two sides of the supraglottis8,22, we decided to check how the myelin fibers are presented in SLN bilaterally and to detect if the myelin fiber distribution pattern is similar or not.
Table 2 demonstrates a statistically significant difference (p<0.001) as compared with the mean age of both groups. The Elderly group is, on average, 31.57 ± 2.9 years older than the Adult group. Therefore, we dealt with an age difference between the groups smaller than that by Mortelliti et al (1990), which was 52.4 years.
Table 3 shows that there is no statistically significant difference in body mass index between the two groups. Mortelliti et al (1990) compared height and weight and found no statistically significant difference between the Young (23.8 years) and the Elderly (76.2 years) groups.
As mentioned above, our sample comprised 11 corpses and we evaluated both sides of the neck with a total of 22 fragments. Each fragment was prepared for each slide, and later assessed by light microscope connected to an image analyzing system LEICA QWINT. The analysis of the myelin fiber morphometric characteristics by means of an automated system makes counting easier and provides accurate and reliable results, particularly when large samples are used and many fields should be assessed19.
Many studies estimated the fiber diameter by measuring all myelin fibers in a cross-section of the nerve. This makes work much more difficult in case of large samples, with a great number of myelin fibers per section, like in SLN, with 3,000 to 5,000 fibers.
Myelin fiber counting was carried out based on a randomized sampling of fields, and at least 6% of the section area of each nerve was assessed. Afterwards, the total frequency of myelin fibers for the total intraperineural area was estimated. Mayhew & Sharma (1984) demonstrated the advantages and limitations of making these measurements by using a semi-automatic counting system. Based on statistical analysis, they concluded that in nerves with a large number of fibers, collection by sampling of 6% of the total area is as accurate as measurement of all myelin fibers. Apart from an efficient evaluation of frequency of fibers, the field sampling is also associated with lower cost and reduced research time, thus reducing likelihood of errors.
We found no statistically significant difference in the intraperineural areas of the Adult and Elderly groups; but we observed a larger intraperineural area on the left side in both groups, which was statistically significant (p=0.008), as demonstrated in Graph 1. Accordingly, the SLN has equal intraperineural area both in Adults and Elderly, and it is larger on the left as compared with the right side. Mortelliti et al (1990) did not find any difference between the areas when comparing diverse age groups. Jacobs & Love (1985) assessed the sural nerve in humans and reported increased section area according to age, as a result of increased connective tissue and greater spacing among the myelin fibers observed in the Elderly.
There was no statistically significant difference between the groups regarding myelin fiber numerical density. We observed that density on the right is greater than on the left side, with statistically significant difference (p=0.043), as shown in Graph 2. Hence, the SLN presents similar density in Adult and Elderly individuals, and it is greater on the right side, as compared with the left side. Mortelliti et al (1990) did not find difference in density considering the diverse age groups.
In the Adult group, the frequency of myelin fibers on the right side varied from 3,552 to 5,499, mean = 4,631 ± 825, and, on the left side, it ranged from 2,546-5,960, mean = 4,985 ± 1,394. In the Elderly group, the frequency of myelin fibers on the right side varied from 3,302 to 4,985, mean = 3,920 ± 623, and, on the left side, it ranged from 3,304-5,386, mean = 4,188 ± 734.
Graph 3 demonstrates a predominance of shorter diameter myelin fibers in both groups. Thus, SLN has shorter diameter myelin fibers (1 and 2 µm), both in Adults and the Elderly, and both groups present a similar distribution as to right and left sides. After statistical analysis of the results of the present study, we verified that the Elderly present lower frequency of short diameter myelin fibers (1 and 2 µm), as compared with younger individuals. According to the literature, the SLN is predominantly composed by smaller myelin fibers (1-2 µm)7,20,21,25.
Evaluating the right SLN, Mortelliti et al (1990) demonstrated statistically significant difference in frequency of smaller myelin fibers in their two groups. Dahlqvist (1982) did not observe difference in frequency of myelin fibers comparing the right and left sides when assessing the RLN in rats. Based on our findings, this pattern also occurs in human SLN.
The diameter of nervous fibers provides a convenient basis to compare fibers among different groups to be studied. The external diameter of fibers has been used to classify the myelin fibers into different groups. It also correlates well with their physiological properties, such as conduction speed of a nervous stimulus23.
The peripheral nerve fibers have variable caliber, ranging from intensively-myelin and large-diameter fibers to very thin fibers with no myelin. The action potential conduction is faster in larger caliber myelin fibers. Nervous stimulus transmission to skeletal muscles is performed by myelin fibers of larger diameter, ranging 8-16µm. The sensorial stimulus transmission could be made by myelin and amyelin fibers27.
According to Burt (1995), the physiologists classify the sensorial nerve fibers into four types:
- Type I: large diameter myelin fibers (12-20 µm), conducting the afferent stimuli from the annular-spiral terminations of muscular fuses and Golgi bodies in tendons.
- Type II: medium-sized diameter myelin fibers (5-12 µm), conducting the afferent stimuli from the "bouquet" terminations of the muscular fuses and of skin tactile receptors.
- Type III: small diameter myelin fibers (1-5 µm), conducting the afferent stimuli related to gross tactile stimulation, temperature and some acute pain sensation.
- Type IV: amyelin fibers (0.5-2 µm), conducting the afferent stimuli related to gross tactile stimulation, temperature and some chronic pain sensation.
The recurrent laryngeal nerve (RLN) is the main responsible for motor innervation of laryngeal intrinsic muscles, and in humans and other animals it is composed primarily by large diameter myelin fibers19,21,26,28. Harrison (1981) assessed the left RLN and observed that large diameter myelin fibers are more frequent, as compared to the right side, and this fact could be related to faster conduction of the nervous impulse, since this nerve has a longer path. Dahlqvist (1982) and Lima (1995) did not find any difference in frequency of myelin fibers in RLN when comparing both sides.
The use of human tissue enables detection of alterations inherent to aging process that we could not observe in animal models with a relatively short survival period. The histomorphometric evaluation of sensorial nerves in other species (rats) did not show statistically significant differences comparing distinct age groups18,25. However, the same study in humans, who have a longer survival, detects some differences.
Jacobs & Love (1985), in a quantitative study about the sural nerve, observed decreased myelin fiber density with aging, associated to increased diameter of fibers, with greater spacing among them and increased cross-section area in the Elderly; moreover, they believed myelin fiber degeneration is a common event in those aged over 60 years.
Taking into account that shorter SLN myelin fibers are functionally sensorial and in charge of supraglottic and subglottic region sensitivity, associated with sudden vocal fold closure reflex and swallowing movements, the reduced number of this type of fiber could be associated with decreased sensitivity in the region, resulting in predisposition to foreign body aspiration and repeated pneumonias in the Elderly.
Comparing our findings with the observations about peripheral nerve physiology mentioned above, we could infer that the Elderly presented decreased sensitivity in the SLN innervated area, since they have smaller amount of myelin fibers, mainly small-sized fibers. Reduced laryngeal sensitivity could also contribute to decreased reflex tonus of the intrinsic muscles and presence of vocal alterations in the Elderly.
These findings should be compared with the clinical alterations observed, such as choking, reduced swallowing speed, penetrations or microaspirations, which are more frequent at this age.
CONCLUSIONS
The histomorphometric study of the SLN in corpses of Elderly individuals as compared to Adults showed similar total number of myelin fibers in both groups. The intraperineural area on the left side is larger than the right side area, but it is compensated by greater density of myelin fibers on the right side.
There is a statistically significant decrease in short diameter myelin fibers (1 and 2 µm) in SLN of the Elderly, if compared with younger individuals.
Combining our findings and observations of other authors we could infer that decreased laryngeal sensitivity could contribute to reduce reflex tonus of the intrinsic laryngeal muscles, presence of vocal alterations and predisposition to dysphagia and aspiration in the Elderly.
Further studies correlating histology and clinical features are needed to confirm or formulate new theories that justify the complaints, very often disregarded, made by Elderly patients and that interfere in their quality of life.
REFERENCES
1. Storey A. A functional analyses of sensory units innervating epiglottis and larynx. Expt. Neurol 1968;20:366-83.
2. Sasaki CT & Weaver EM. Physiology of the larynx. Am J Med 1997;103(5A):9s-18s.
3. Bradley RM. Sensory receptors of the larynx. Am J Med 2000;108(4A):47s-50s.
4. Sanders I, Wu BL, Mu L, Li Y, Biller HF. The innervation of the human larynx. Arch Otolaryngol Head Neck Surg 1993;119:934-39.
5. Wu BL, Sanders I, Mu L, Biller HF. The human communicating nerve: an extension of the external superior laryngeal nerve that innervates the vocal cord. Arch Otolaryngol Head Neck Surg 1994;120:1321-27.
6. Suzuki M. Laryngeal reflexes. In: Hirano M, Kirchner JA, Bless D. Neurolaryngology: recent advances. San Diego: Singular Publishing Group; 1991. p. 142-55.
7. Mortelliti AJ, Malmgreen LT, Gacek RR. Ultrastructural changes with age in the human superior laryngeal nerve. Arch Otolaryngol Head Neck Surg 1990;116:1062-69.
8. Aviv JE, Martin JH, Jones ME, Wee TA, Diamond B, Keen MS, Blitzer A. Age-related changes in pharyngeal and supraglottic sensation. Ann Otol Rhinol Laryngol 1994;103:749-52.
9. Kirchner JA. Laryngeal proprioception. In: Hirano M, Kirchner JA, Bless D. Neurolaryngology: recent advances. San Diego: Singular Publishing Group; 1991. p. 156-59.
10. Hirano M, Kurita S, Sakaguchi S. Ageing of the vibratory tissue of human vocal folds. Acta Otolaryngol 1989 (Stockh) 107:428-33.
11. Sato K & Hirano M. Age-related changes of elastic fibers in the superficial layer of the lamina propria of vocal folds. Ann Otol Rhinol Laryngol 1997;106:44-48.
12. Durham CF & Harrison TS. The surgical anatomy of the superior laryngeal nerve. Surg Gynecol Obstet 1964;118:38-44.
13. Cernea CR, Nishio S, Hojaij FC. Identification of the external branch of the laryngeal nerve (EBSLN) in large goiters. Am J Otolaryngol 1995;16:307-11.
14. Rassekh CH, Driscoll BP, Seikaly H, Laccourreye O, Calhoun KH, Weinstein GS. Preservation of the superior laryngeal nerve in supraglottic and supracricoid partial laryngectomy. Laryngoscope 1998;108:445-47.
15. Dyck PJ. Hypoxic neuropathy: Does hypoxya play a role in diabetic neuropathy? Neurology 1989;39:111-18.
16. Karnovsky MJ. A formaldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 1965;27:137.
17. Gundersen HJG. Notes on the estimation of the numerical density of arbitrary profiles: the edge effect. J Microsc 1977;111 pt 2:219-23.
18. Sharma AK, Bajada S, Thomas PK. Age changes in the tibial and plantar nerves of the rat. J Anat 1980;130:417-28.
19. Harrison DFN. Fiber size frequency in the recurrent laryngeal nerves of man and giraffe. Acta Otolaryngol 1981;91:383-89.
20. Ogura JH & Lam RL. Anatomical and physiological correlations on stimulating the human superior laryngeal nerve. Laryngoscope 1953;63: 947-59.
21. Scheur JL. Fiber size frequency distribution in normal laryngeal nerves. J Anat Lond 1964;98: 99-104.
22. Aviv JE. Effects of aging of the pharyngeal and supraglottic areas. Am J Med 1997;103(5A):74s-76s.
23. Mayhew TM & Sharma AK. Sampling schemes for estimating nerve fiber size: I. Methods for nerve trunks of mixed fascicularity. J Anat 1984;139:45-58.
24. Jacobs JM & Love S. Qualitative and quantitative morphology of human sural nerve at different ages. Brain 1985;108:897-924.
25. Rosemberg SI, Malmgreen LT, Woo P. Age-related changes in the internal branch of the rat superior laryngeal nerve. Arch Otolaryngol Head Neck Surg 1989;115:78-86.
26. Dahlqvist A, Carlso B, Hellström S. Fiber components of the laryngeal nerve of the rat: a study by light and electron microscopy. Anat Rec 1982;204:365-70.
27. Burt AM. Neuroanatomia. Rio de Janeiro: Ed. Guanabara-Koogan; 1995.
28. Lima NTMS. Estudo morfométrico das fibras mielínicas do nervo laríngeo recorrente. [Tese Mestrado] USP/Ribeirão Preto; 1995.
1 Master in Otorhinolaryngology and Head and Neck Surgery, Federal University of São Paulo - Escola Paulista de Medicina.
2 Joint Professor, Discipline of Otoneurology, Federal University of São Paulo - Escola Paulista de Medicina.
3 Joint Professor, Center of Electron Microscopy, Federal University of São Paulo - Escola Paulista de Medicina.
4 Joint Professor, Discipline of Otorhinolaryngology, Federal Universidade of São Paulo - Escola Paulista de Medicina.
Affiliation: Department of Otorhinolaryngology and Human Communication Disorders and Center of Electron Microscopy (CEME), Federal University of São Paulo - Escola Paulista de Medicina.
Address correspondence to: Romualdo S. L. Tiago. Rua Estado de Israel, 493, apto 51 - Vila Clementino - São Paulo - CEP 04022-001 - Tel. (55 11) 5084.7725. - E-mail: romualdotiago@uol.com.br
Study presented at II Congresso Triológico de Otorrinolaringologia, held in Goiânia-GO in August 2001, awarded as the best study in Laryngology and Voice.
Article submitted on January 30, 2002. Article accepted on February 10, 2002.