Year: 2003 Vol. 69 Ed. 2 - (17º)
Artigo Original
Pages: 256 to 259
Conservative treatment of Gradenigo's syndrome
Author(s):
Felippe Felix[1],
Maria Cecília Domingues de Olivaes[2],
Ronaldo A. O. C. Gismondi[3],
Heraldo Belmont[4],
José A. de P. Felix[5]
Keywords: Gradenigo's syndrome, petrositis, abducens palsy.
Abstract:
Gradenigo's syndrome, a rare complication of otitis media, consists of suppurative otitis, abducens nerve palsy and pain in the trigeminal nerve area. The occurrence of this disease is associated with osteitis of petrous apex of temporal bone and the neighbor structures. The authors describe in this article the case of a 7 year-old boy with this syndrome treated successfully with conservative treatment instead of surgical treatment.
![]()
Introduction
In the pre-antibiotic era, the dissemination of an infection from the middle ear to the mastoid and petrous bone frequently resulted in serious intracranial complications, such as cavernous sinus thrombosis, meningitis and death. With the advent of new diagnostic techniques and potent antibiotics, such complications are becoming rare, since they are normally interrupted in initial stages. In the present article, we will report a case and discuss Gradenigo's syndrome.
The syndrome was described for the first time in 1907 by Giuseppe Gradenigo (1859-1926) and consisted of the triad of complicated otitis media with purulent otorrhea, pain on the innervation area of the first and second branch of the trigeminal nerve and abducens nerve palsy. The cause may be explained by dissemination of the infection by contiguity from the middle ear to the apex of the petrous portion of the temporal bone through the aired bone cells, located close to the area of the abducens and trigeminal nerve passage.
Treatment to such affection is an important controversy in the world literature, as many authors advocate early surgical intervention, whereas few others believe that conservative treatment manages to resolve signs and symptoms of the syndrome and minimize the onset of sequelae.
In the present article we tried to report a case of Gradenigo's syndrome and discuss the topic emphasizing the adopted treatment and other possible alternatives by means of reviewing the literature.
Case Report
Male 7y10m subject, presented initial clinical manifestation of fever, clear rhinorrhea, and otalgia on the right, progressing for 6 days after marked headache on the right, high fever, vomiting and purulent otorrhea on the right, associated with diplopia. At the end of the week, he came for medical treatment in our center.
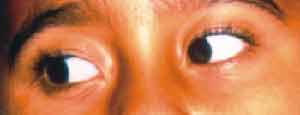
The physical examination showed a patient with fair general status, low fever, neck rigidity, paralysis of the 6th cranial nerve on the right (Figure 1) and pain on the nerve area of the ophthalmic branch of the trigeminal nerve on the right: there were no phlogistic signs in the mastoids. Left otoscopy was normal and on the right there was hyperemia of tympanic membrane.
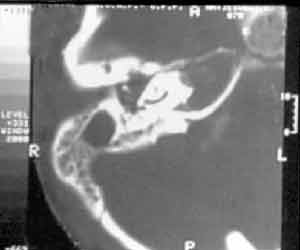
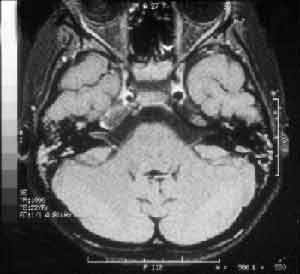
Head computed tomography (CT) presented velamentum of bilateral mastoid cells, more marked on the right, compatible with acute mastoiditis; there were no signs of cerebral abscess (Figure 2). The CSF analysis showed 10 cel/mm3 (100% polymorphonuclear), 51 mg/dl of proteins and 66 mg/dl of glucose; the culture was negative. Right ear secretion swab showed in the direct exam the presence of gram positive diplococcus and some gram-positive coccus, and in the culture it revealed alpha-hemolytic Streptococcus. Magnetic resonance imaging (MRI) confirmed the findings of acute mastoiditis and revealed temporal petrositis on the right (Figure 3). Based on these clinical and radiographic findings we diagnosed the case as Gradenigo's syndrome.
The patient was hospitalized and we started with venous antibiotic therapy, initially with oxacyllin and ceftriaxone, replaced 7 days later by cefpime, which was maintained for 21 days, comprising a total of 28 days of therapy. On the second day with antibiotics, there was remission of headache and neck rigidity. During the hospitalization, the patient presented worsening of nausea and vomiting, and abdominal pain on the right superior quadrant. Lab tests, including serology, confirmed acute hepatitis A.
After 28 days of hospitalization, the patient was asymptomatic, except for the 6th cranial nerve paralysis on the right. The CT scan was normal and the patient was discharged with amoxicyllin and clavulanate for 7 more days.
In the outpatient follow-up, 3 weeks after discharge, there was complete remission of 6th cranial nerve paralysis on the right (Figure 4). The patient repeated MRI 2 months after discharge and the result was normal.
Figure 1. Patient looking to the right; note the right eye does not follow the left eye movement.
Figure 2. CT with right mastoid velamentum, tissues with soft tissue density occupying the external auditory canal and middle ear.
Figure 3. MRI with liquid in mastoid cells and middle ear bilaterally, reduction of the right internal carotid artery caliper in its intracavernous portion.
Figure 4. Patient looking to the right; note the improvement of the abducens nerve palsy on the right.
Discussion
The patient in our study presented classical signs and symptoms of Gradenigo's syndrome whose etiopathogenesis can be understood by the description of the anatomy of the temporal bone petrous portion. The area has a pyramidal shape; on the basis, lie the mastoid and the middle ear and on the apex it is separated by the dura mater from the trigeminal ganglion and Dorello's canal, where the abducens nerve passes. The dissemination of the basis to the top occurs through the aired cells; in 80% of the people they are found in the mastoid and in only in 30%, in the apex of the petrous portion1.
In past decades, there has been osteitis and consequent leptomeningitis, leading to cranial nerve disorders by proximity of the nervous structures referred. However, there is a report of petrous osteitis of the non-pneumatized temporal bones with likely direct dissemination of the infection by osteomyelitis, facial plans and vascular canals2.
The interval from the beginning of otitis up to comprise of cranial nerves can vary from 1 week, as in the case reported, to 2 to 3 months3. The most common pathogens are hemolytic streptococcus and pneumococcus 4, but some authors reported great frequency of Staphylococcus aureus and in some chronic cases, Pseudomonas aeruginosa1. Most of the patients present with hyperesthesia of the trigeminal area innervation, especially of the ophthalmic branch, diplopia and evidence of middle ear infection.
Petrositis can be classified into acute and chronic, according to its clinical course and severity5, 6. The acute form is characterized by unspecific infection with inflammatory process that involves mucosa and bone tissue, with similar findings in the middle ear and mastoid, whereas the chronic form is marked by thickness of the mucosa membrane of the petrous bone as a result of fibrous proliferation. Frequently, in the latter, there is a cystic center space surrounded by thick mucosa involved in bone tissue. Such areas lead to entrapment of the infection of the chronic process, making it difficult to be treated only with antimicrobial therapy.
The advances in radiology provided easy diagnosis of petrous apex affection, whereas the development of antibiotics made them even rarer. Computed tomography scan7, 8 can show velamentum of the air cells and periosteal involvement with erosion in the petrous apex. Whether there is presence or not of air cells close to the apex of the petrous portion does not exclude the impairment, as preciously discussed. Magnetic resonance imaging2, 9 is useful to assess soft tissue lesions, showing in most times, hypointense image in T1 in the petrous apex topography, hyperintense image in T2 and greater enhance in the region with use of venous contrast (gadolinium). In our case, there was evidence of affection of the cavernous sinus showing narrowing of the carotid artery in the intracavernous portion (Figure 3).
Differential diagnosis should be made with otic hydrocephalus10, intracerebral abscesses and petrous apex tumors (such as meningioma, neuroma or metastases), cholesteatoma and intracranial aneurysm3.
Among the possible complications11, 12, we can include meningitis, affection of other cranial nerves (such as facial and trochlear), intracranial abscess, parapharyngeal/pre-vertebral abscess, dissemination by the sympathetic plexus around the carotid artery (leading to Claude-Bernard-Horner syndrome) and dissemination of the skull base leading to affection of 9th, 10th and 11th cranial nerves (Vernet's syndrome). Severe cases can evolve with hemorrhage by lesion of the carotid artery wall and/or thrombosis of basal venous sinuses3.
Historically, the treatment of petrositis has been surgical intervention13. However, some situations have allowed the option for a more conservative treatment, especially when there is no signs of chronic otitis. This form of treatment for acute petrous osteitis has shown good results with the use of wide spectrum antibiotics and good intracranial penetration, associated with myringotomy. In the case reported here, we started with ceftriaxone with oxacyllin, replaced 7 days later by cefpime owing to the picture of acute hepatitis and possible hepatotoxicity of oxacyllin, maintained up to 4 weeks of venous antibiotic therapy. At the time of discharge, with no signs of infection, we maintained 7 more days of oral antibiotic therapy, using amoxicyllin/clavulanate. We excluded the possibility of conducting a myringotomy or any surgical intervention in the case. The most aggressive treatment with mastoidectomy is reserved for patients that are refractory to conservative therapy or that demonstrate signs of chronic processes13.
In the literature, we found few reports of conservative treatment. Minnoti14, in 1999, used myringotomy as treatment, venous antibiotic therapy for 4 days (with improvement of 6th cranial nerve paralysis in the end), and oral treatment for 3 more weeks. Marianowski15, in 2001, reported myringotomy with venous antibiotic therapy for 33 days (ceftriaxone, metronidazole and amykacin, the latter for only 3 days), confirming the total improvement of abducens nerve paralysis after approximately 60 days from beginning of treatment. Tutuncuoglu16 (1993) reported the use of ceftriaxone and metronidazole for 2 weeks followed by cotrimoxazole for 6 more weeks, with improvement of paralysis 3.5 months later. The improvement in trigeminal nerve pain occurred always within the first week after the beginning of intravenous antibiotic therapy. In the case presented here, the improvement in facial pain, presented as headache, happened 2 days after use of antibiotic therapy and the abducens nerve paralysis was completely solved 48 days after the beginning of treatment.
CLOSING REMARKS
Gradenigo's syndrome, despite its rare occurrence after the advent of antibiotics, should be considered when there is facial pain and abducens nerve paralysis before otitis media pictures. Its classical approach consists of aggressive surgical intervention. However, new reports have described good results with conservative treatment, reserving surgery for chronic cases or those refractory to clinical therapy.
References
1. Gillanders D. Gradenigo's Syndrome Revisited. J Otolaryngol 1983;12(3):169-74.
2. Hardjasudarma M, Edwards R, Ganley J, Aarstad R. Magnetic resonance imaging features of Gradenigo's syndrome. Am J Otolaryngol 1995;16(4):247-50.
3. Graaf J, Cats H, de Jager A. Gradenigo's syndrome: a rare complication of otitis media. Clin Neurol Neurosurg 1988;90(3):237-9.
4. Utrata J. Gradenigo's syndrome - bilateral occurence. Eye Ear Nose Throat Mon 1973;52:54-6.
5. Allam A, Schucknecht H. Pathology of Petrositis. Laryngoscope 1968;78:1813-32.
6. Chole R., Donald P. Petrous Apicitis: Clinical Considerations. Ann Otol Rhinol Laryngol 1983;92:544-51.
7. Murakami T, Tsubaki J, Tahara Y, Nagashima T. Gradenigo's syndrome: CT and MRI findings. Pediatr Radiol 1996;26:684-5.
8. Woody R. The role of Computerized Tomographic scan in the management of Gradenigo's syndrome: a case report. Pediatr Infect Dis 1984;3(6):595-7.
9. Davé A, Diaz-Marchan P, Lee A. Clinical and Magnetic Ressonance Imaging Features of Gradenigo Syndrome. Am J Ophtalmol 1997;124(4):568-70.
10. Homer J, Johnson I, Jones N. Middle ear infection and sixth nerve palsy. The Journal of Laryngology and Otology 1996;110:872-4.
11. Frigola J, Vazquez C, Valdes-Hevia J, Perez A, Hernandez M. Síndrome de Gradenigo. A Propósito de um Caso Pediátrico. An Esp Pediatr 1992;36(2):151-2.
12. Motamed M, Kalan A. Gradenigo's syndrome. Postgrad Med J 2000;76:559-60.
13. Al-Ammar A. Recurrent Temporal Petrositis. The Journal of Laryngology & Otology 2001;115:316-8.
14. Minotti A, Kountakis S. Management of abducens palsy in patients with petrositis. Ann Otol Rhinol Laryngol 1999;108:897-902.
15. Marianowski R, Rocton S, Aimer J, Morrisseau-Durand M, Manach Y. Conservative management of Gradenigo syndrome in a child. Int J Pediatr Otorhinolaryngol 2001;57(1):79-83.
16. Tutuncuoglu S, UranN, Kavas I, Ozgur T. Gradenigo syndrome: a case report. Pediatr Radiol 1993;23:556.
1 Undergraduate, Medical School, Universidade Federal Fluminense.
2 Joint Professor, Discipline of Pediatric, Medical School, Universidade Federal Fluminense.
3 Undergraduate, Medical School, Universidade Federal Fluminense.
4 Head of the Service of Radiology, Hospital da Casa de Portugal.
5 Joint Professor, Discipline of Otorhinolaryngology, Medical School, Universidade Federal Fluminense.
Study conducted at University Hospital Antonio Pedro, Universidade Federal Fluminense.
Address correspondence to: Felippe Felix - R.Cel. Moreira César 229 sala 1815
Icaraí - Niterói RJ 24230-053
Tel/Fax (55 21) 710-6220 - E-mail: felfelix@cruiser.com.br
Article submitted on February 26, 2002. Article accepted on April 11, 2002.