Year: 2003 Vol. 69 Ed. 2 - (10º)
Artigo Original
Pages: 208 to 213
Anatomic description of the presence of zinc in cochlear nuclei
Author(s):
Profª. Dra. Maria Cristina L. C. Féres[1],
Prof. Dr. Norberto G. Cairasco[2]
Keywords: zinc, cochlear nuclei, histochemistry.
Abstract:
The presence of zinc has been noticed in different areas of the central nervous system, and, in the auditory system, cochlear nuclei. Its physiological role has not yet been fully described. This work has as main goals: to study the anatomic distribution of zinc, by means of histochemistry, and to compare it with the morphological features observed by using Nissl-staining methods, in cochlear nuclei. The subjects were adults rats. They were sacrificed by intracardiac perfusion, following a protocol for zinc histochemistry. The cochlear nuclei were studied by light microscopy. The authors describe the differenciated distribution of zinc among dorsal and ventral cochlear nuclei. They concluded that this feature has a relationship with the physiological differentiation of them.
![]()
INTRODUCTION
Cochlear nuclei (NNCC) are the first stop of the auditory pathways into the central nervous system, comprising neurons classified as "secondary", which receive ascending information from the cochlear nerve fibers (NeC)1. Its basic position and organization is very uniform in rats, cats, rabbits and mice, with slight differences compared to primates2. They are located on the lateral borders of the junction between the pons and the bulb, in the brainstem3. Classically, they are subdivided into dorsal nucleus (NCD), ventral nucleus, which can be subdivided into anterior (NCVA) and posterior nucleus (NCVP), and interstitial nucleus (NI) or root of the cochlear nerve. The axons of NeC get into the NI and bifurcate into ascending and descending segments. The subdivision of the ventral nucleus into anterior and posterior is relative to the NeC entry point. The ascending branch penetrates into the NCVA arranged in fascicles, which are maintained fixed. The descending branch goes into the NCVP, also arranged in fascicles, and then gathers in a single bundle and directs towards the NCD3.
The projection of NeC fibers, from NNCC entry, follows a regular scheme, originating the cochleotopic representation, that is, respecting the origin of fibers in the cochlea and allowing a more precise distribution of frequencies in the NNCC 4. The nervous fibers coming from neurons with basal position in the cochlea followed the most dorsal regions of the NNCC. Fibers coming from apical regions reach preferably ventral positions1, 4. In cats, the destruction of the cochlear basal portion (site of high frequency perception) leads to degeneration of the dorsal portions of the NCV. Destruction of the apical cochlear area (low frequencies) leads to ventral degeneration of the same nucleus3.
Current knowledge shows that NNCC perform a highly differentiated task in treating and integrating auditory messages, which can not be considered as mere signal passage stations. It has a very complex structural organization, containing different neuronal populations, with high levels of fibers coming from the central regions of the auditory pathways and provides a great variety of intranuclear circuits. There seems to be a distinction between the dorsal and ventral subnuclei functions, because the former modulates the latter4, 5.
As of the second half of the century, the use of transition metals in biological reactions became more common. Such metals became part of the structure of some enzymatic and non-enzymatic proteins in addition to binding to some other types of biological molecules. Chemical analyses showed the presence of metals in the central nervous system (CNS), requiring the development of investigation protocols to improve the knowledge about its distribution in tissues and cells, using histochemical techniques to do that. In the second half of the century, studies using a chelating agent, dithizone, revealed the presence of zinc in the hippocampus. In 1958, Timm demonstrated the same fact through histochemical development with silver sulfate. Other studies followed it, finding a marked presence of zinc, especially in mossy fibers of the hippocampus. 6, 7
It is known that zinc, in addition to being an essential nutritional component for vegetal and animal metabolism has a very specific function in excitatory central nervous transmission in mammals8. The cation is found in some sympathetic terminals, sequestrated from the axonal buttons, released in the sympathetic fissure after the onset of the electrical impulse, such as a probable mechanism of exocytosis of the vesicles that contain it. This mechanism depends on calcium. Initially detected in the limbic system structures (hippocampus and amygdala),it is currently described in layers 1-3 and 5 of the cerebral cortex, pineal gland and also cochlear nuclei8, 9, 10.
Its physiological role is not well known. Once the neural routes in which it was identified are glutamatergic routes and knowing that zinc inhibits the bond of glutamate to its receptors, especially N-Methyl-D-Aspartate (NMDA), it is believed that it plays a role in the modulation of this type of synapses9. The increase in concentration of extracellular zinc, owing to successive sympathetic and excitatory activities, would lead to post-synaptic receptor block, being thus, responsible for the functional control of the system. The function of the ion seems to be that of protecting against neuro-excitatory effects8. An antagonist effect of zinc over GABA receptors has also been demonstrated 11.
In some regions, the ion is bond to pro-proteins, stabilizing them. It is attached to a substance named neuronal growth factor - NGF, especially to a storing complex, the 7-NGF. It is a neurotrophic factor whose activation suggests an afferent loss of its systems and it is connected to the occurrence of the plastic phenomenon of neural sprouting. It is known that the hippocampus is very rich in NGF and that zinc seems to operate as a regulator of the trophic phenomenon secondary to the loss of afferent fibers, in addition to being a good histochemical marker9, 22.
The location of zinc in the cochlear nuclei, according to Frederickson et al. (1988)9, can be made by two different formats: the former is presented as a cover along the whole surface of the dorsal nucleus, corresponding to its molecular layer. In the second type, zinc has a spotted shape, in the regions that correspond to the granular cells. Such well-differentiated location makes the ion a good marker for histochemical studies of the abnormalities of the neural circuit of the cochlear nuclei. The literature, however, fails to demonstrate in details the distribution of zinc along the anterior-posterior extension of the cochlear nuclei and its correlation with the physiology of the subgroups.
The purposes of the present study were:
1. To study in detail the availability of zinc along the whole extension of the cochlear nuclei.
2. To compare the distribution with the anatomical subdivision of the nuclei, studied by Nissl staining.
MATERIAL AND METHODS
Eleven albino adult Wistar rats, aged 70 days of life, underwent the present study.
The animals were sacrificed by intracardial perfusion, following the specific protocol for the histochemical technique of Neo-Timm. Since this protocol is not in conflict with the Nissl staining, it was possible to use the same subjects for the study with both techniques.
Initially, the animas received an intraperitoneal injection of sodium selenite solution in distilled water, concentration of 20mg/ml, dose of 20mg/Kg. Sodium seletine has a chelating action over tissue zinc, precipitating it and maximizing the ion by the Neo-Timm9 protocol. Thirty minutes after the application of the selenite, we conducted the perfusion. We initially made the perfusion of 100ml of buffer solution Millonigs 0.12 M, pH 7.4. Next, we introduced 100ml of sodium sulfite solution at 0.1% in the same buffer. The sulfite reacted with zinc, precipitating it in the tissues. Next, we administered 100ml of glutaldehyde at 3% in bi-distilled and deonized waster for fixation and again sulfite at 0.1% in Millonigs, in 200 ml volume. The removed brains were stored during the night in sacarose solution at 15% in glutaldehyde at 3%. Next, we conducted freezing with 2-methylbutane at 99.5% (Sigma-Aldrich), at -40ºC.
The brains were sectioned with the cryostat (Micron-Zeiss), in 25mm sections, placed on gel glass slides. The sections comprised the extension of the cochlear nuclei, between 11.60 and 9.68mm in the anterior-posterior axis, as of the Bregma, according to the stereotaxic atlas by Paxinos and Watson (1986)13. For each level, a slide was sent to Nissl staining study, one to Neo-Timm histochemistry, and one for storage.
The development of the Neo-Timm technique is similar to that of a photo development, in which we use a silver nitrate bath6, 7. Silver reacts with the precipitated zinc from the tissues by the sulfite and selenite, forming a visible brown compound, which can vary color according to the amount of zinc present in tissues and the duration of exposure in the bath.
After the preparation and fixing of the slides, they were studied under optic microscopy and we screened NNCC from anterior to posterior direction, analyzing the distribution of zinc labeling.
Zinc precipitated from the tissues, when developed by the Neo-Timm histochemistry, takes on a brown color and the intensity is directly proportional to the ion concentration. Thus, regions with high concentrations of zinc are strongly stained in brown. Regions with low concentrations of zinc are light brown. After the transformation of the images into gray scale, there is a proportional correlation between gray intensity and concentration of ions in the tissues.
RESULTS
The distribution of zinc in the cochlear nuclei of the different sections is shown in the figures below.
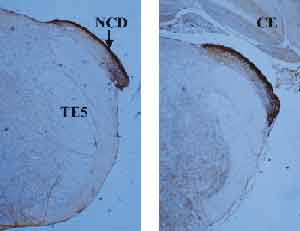
Figure 1 shows the cochlear nucleus in its posterior end, in which there is a dorsal sub-nucleus. It is important to point out that the dorsal and ventral sub-nuclei are not totally coinciding on the sagittal plan. The dorsal cochlear nucleus is located more posteriorly, whereas the ventral one is more anterior. The picture shows that the dorsal cochlear nucleus has strong zinc, especially on the most external layer, named molecular layer. In the molecular layer, there are small rounded abundant cells named granular cells. This type of cell is also found in the cerebellum and hippocampus, regions that are also rich in zinc.
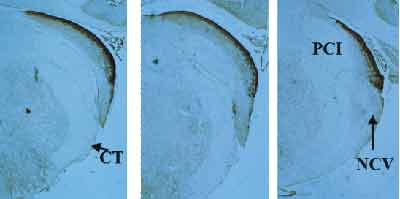
Figure 2 shows intermediate sections, in which we visualize the dorsal and ventral sub-nuclei. As the sections progress anteriorly, the dorsal nucleus reduced its projection, whereas the ventral one became more evident. Note that zinc labeling in the ventral cochlear nucleus is less intense, slightly spread by the interior of the nucleus and forming a thin layer that is more concentrated in the periphery of the tissue.
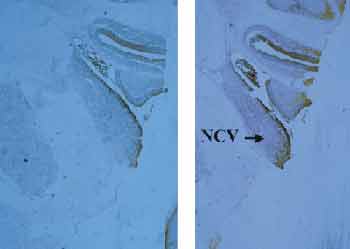
In Figure 3, we can see more anterior sections, in which only the ventral cochlear nucleus is located, with a histochemical labeling pattern described in the previous paragraph.
Figure 4 emphasizes the limits between dorsal and ventral pattern, in which we can see that all studied sections have a enhancement of the zinc labeling pattern. Upon the study of this region with Nissl staining, for cell evidencing, we noticed marked concentration of granular cells, corresponding to the same cell pattern of other zinc rich regions.
Figure 1. Sections of the posterior extremity of the cochlear nuclei, evidencing only the dorsal cochlear nucleus, richly marked with zinc by histochemistry. NCD = dorsal cochlear nucleus; CE = cerebellum; PCI = inferior cerebral pedunculum; TE5 = trigeminal spinal tract.
Figure 2. Note the gradual onset of the ventral cochlear nucleus as the level of the section advances towards the anterior region, with histochemical labeling that is less intense than in the dorsal region. NCV = ventral cochlear nucleus. PCI = inferior cerebral pedunculum; CT = corpus trapezoideum.
Figure 3. Anterior extremity of the cochlear nucleus, in which we can find the ventral sub-nucleus, with mild staining for zinc, showing lower concentration of the ion at this level of the nucleus. NCV = ventral cochlear nucleus.
Figure 4. Transition between dorsal and ventral cochlear nuclei, in which there is a stress of the histochemial labeling pattern. Note that in B and C, there is a high concentration of granular cells (arrows). B = 40x magnification; C = 100x magnification.
DISCUSSION
The Neo-Timm method proved to be very efficient for use in cochlear nuclei. Initially described by Timm, in 19586, and later modified by Danscher (1981)14, it consists of zinc development with silver nitrate, forming dark visible granules at optic microscopy. The first studies located zinc in the synaptic buttons of the mossy terminal fibers, which are axons of the granular cells of the hippocampus denteate gyres, but subsequent studies confirmed that the ion was present in other synaptic systems, such as the cochlear nuclei6, 7. Frederickson et al. (1988)9 described a differentiated distribution of zinc labeling in NNCC. The first one is more diffuse and spotted along the regions of granular cells; the second one is more compact and located on the molecular layer, which is the most external layer of NCD. The same was confirmed by Haug (1973)6.
In our study, cochlear nuclei showed a distribution of zinc labeling that was very similar to the description below. In NCD, the labeling was more marked, delimiting a more or less thick layer on the dorsal aspect of the nucleus. More internally, the labeling is of spotted less dense pattern. In NCV, zinc formed a thin layer on the dorsal surface; in the anterior regions of the nucleus, the zinc-labeled layer was located towards the ventral-medial direction, following the contours of the nucleus and delimiting it form the anterior cerebral pedunculum. Inside the NCV, the labeling pattern was also scarce spotted. In the interface between the dorsal and ventral cochlear nuclei, there is an invagination of granular cells, clearly labeling the limit between them. This region is also strongly labeled for zinc, as shown in Figure 3.
The origin of the zinc evidenced in the cochlear nuclei is not confirmed. In the molecular layer of the NCD, it is believed that it is present in the synapses originated from the parallel fibers, which are the axons of granular cells. Previous observations in prosencephalic structures located zinc as a co-factor in glutamatergic synapses. Studies showed that granular cells of the cochlear nuclei use the glutamate as a neurotransmitter15. It is known that parallel fibers make innumerous passage synapses along the molecular layer. The labeling pattern in granules can correspond to such synapses points8, 9.
The labeling pattern found in the center of the nuclei and in other areas with granular cells can represent either the axons of these cells or the mossy fibers, which make synapses in glomeruli with the dendrites of granular cells and Golgi cells associated with them. The fact that it is a more scarce labeling make us consider that if mossy fibers use zinc as a co-factor, it is not present in all the cells9.
The type of afferent system received by granular cells is not completely known. Studies pointed towards fibers of the olivocochlear bundle or other descending bundles, which penetrate into the cochlear nuclei and positively label acetylcholine; others point towards the afferent fibers as being the origin of mossy fibers4, 16. If this is truth, then the zinc sparsely found in the areas dominated by the granular cells does not come from the mossy fibers, since they do not use glutamate as a neurotransmitter. Most probably, it is located in synaptic buttons of granular cell axons. Another fact that has been described is the entry of type II cochlear neuron fibers in synapses with the granular cells of cochlear nuclei17, and as indicated so far, such synapses used glutamate as neurotransmitter4.
It seems, therefore, that zinc participates in the synaptic transmission mechanism related to the operating of granular cells. The way such cells participate in the circuit of the acoustic pathways is still not completely known. It is known that the characteristics of Nissl staining reminds us of the cerebellum granular cells and it is believed that there could be some similarity between both also from a functional perspective. The distribution of granular cells in NCD and NCV and the existence of parallel fibers crossing the molecular layer of NCD, makes us wonder about a direct action of this type of cells on the operation of the molecular layer16. Since granular cells are interneurons whose afferent paths are received from modulators of the auditory system, we can state that zinc, since it is a synaptic modulator itself, has an essential role in the control of the functional system. It is known that NCD afferent path reaches the neurons in the NCV, whose function is to conduct information coming from the peripheral area of the rostral structures relative to the auditory pathways. In such case, we can assume that NCD exercises, by means of such afferent path, a control over the passage of signal sent by the Central Nervous System. The fact that NCD is rich in zinc, which is suspected to have a synapses modulating role, and the receiving of a rich innervation from the efferent auditory system, contribute to reinforce this concept.
CONCLUSIONS
1. Study conducted with Nissl staining and Neo-Timm histochemical technique evidenced the anatomical structure of the dorsal and ventral cochlear nuclei, as well as the differentiated distribution of zinc-rich systems.
2. The use of the Neo-Timm technique to detect zinc in tissues allow the study of the anatomical distribution of some neural circuits in the cochlear nuclei, which can be used to study such structures in normal conditions and in plastic rearrangements.
3. The differentiated distribution of zinc in dorsal and ventral cochlear nuclei reinforced the concept of having different physiological roles for both structures.
4. The dorsal nucleus bond with the efferent auditory system, the presence of zinc ion in large amounts and the existence of fibers that cross the dorsal nucleus to the ventral one, are strong evidence that such sub-nucleus plays an important modulating role in the beginning of the central auditory pathway.
REFERENCES
1. Rouiller EM. Organisation fonctionnelle des voies auditives. In : Romand, R Le Systeme Auditif Central. Paris: Les Éditions Inserm; 1992. p.1-49.
2. Harrison JM, Warr WB. A study of the cochlear nuclei and ascending auditory pathways of the medulla. J Comp Neurol 1962;119:341-352.
3. Webster WR. Auditoy System. In: Paxinos (Ed). The Rat Nervous System. Academic Press Inc.;1995. p.797-831.
4. Romand R. Aspects anatomo-fonctionnels du noyau cochléaire. In: Romand R. (Ed) Le systeme auditif central. Anatomie et physiologie. Paris: Les Éditions Inserm; 1992. p.51-108.
5. Brawer DK, Kane EC. The neuronal architecture of the cochlear nucleus of the cat. J Comp Neurol 1974;155:251-300.
6. Haug FMS. Heavy metals in the brain. Adv Anat Embryol Cell Biol 1973;47 (4):04-71.
7. Danscher G, Howell G, Pérez-Clausell J, Hertel N. The dithizone, Timm's sulphide silver and the selenium methods demonstrate a chelatable pool of zinc in CNS. Histochemistry 1985;83:419-422.
8. Peters S, Koh J, Choi DW. Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons. Science 1987;236:589-593.
9. Frederickson CJ, Howell GA, Haigh MD, Danscher G. Zinc-containing fiber systems in the cochlear nuclei of the rat and mouse. Hear Res 1988;36:203-212.
10. Howell GA, Perez-Clausell J, Frederickson CJ. Zinc containing projections to the bed nucleus of the stria terminalis. Brain Res 1991;562:181-189.
11. Christensen MK, Geneser FA. Distribution of neurons of origin of zinc-containing projections in the amygdala of the rat. Anat Embryol 1995;191:227-237.
12. Stewart GR, Frederickson CJ, Howell GA, Gage FH. Cholinergic denervation-induced increase of chelatable zinc in mossy-fiber region of the hippocampal formation. Brain Res 1984;290:43-51.
13. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, Second Edition. Academic Press Inc.; 1986.
14. Danscher G. Histochemical demonstration of heavy metals. A revised version of the sulphide silver method suitable for both light and eletronmicroscopy. Histochemistry 1981;71:1-16.
15. Wenthold RJ, Hunte C, Petralia RS. Excitatory amino acid receptors in the rat cochlear nucleus. In: Merchán M, Juiz J, Godfrey D, Mugnaini E (Eds). The mammalian cochlear nuclei. Organization and function. New York and London: Plenum Press; 1993. p.179-194.
16. Mugnaini E, Warr WB, Osen KK. Distribution and light microscopic features of granule cells in the cochlear nuclei of cat, rat and mouse. J Comp Neurol 1980;191:581-606.
17. Brown MC. Anatomical and physiological studies of type I and type II spiral ganglion neurons. In: Merchán MA, Juiz JM, Godfrey DA, Mugnaini E (Eds). The Mammalian Cochlear Nuclei. New York and London: Plenum Press; 1993. p.43-54.
1 Ph.D., Professor, Discipline of Otorhinolaryngology, Medical School, Ribeirão Preto, University of São Paulo.
2 Ph.D., Professor, Head of the Neurophysiology and Experimental Neurootology Laboratories,
Department of Physiology, Medical School, Ribeirão Preto, University of São Paulo.
Address correspondence to: Profa. Dra. Maria Cristina Lancia Cury Féres
Departamento de Oftalmologia, Otorrinolaringologia e Cirurgia de Cabeça e Pescoço -
Avenida Bandeirantes, 3900 12º andar 14049-900 Monte Alegre Ribeirão Preto SP.
Tel (55 16) 602-2863/ 602-2526 - Fax (55 16) 602-2860 - E-mail: mcferes@hcrp.fmrp.usp.br
Stud7 presented at the XXXVI Congresso Brasileiro de Otorrinolaringologia, in Florianopolis, November 2002,
Awarded Free Communication, financially supported by FAPESP, CAPES, CNPq.
Article submitted on December 13, 2002. Article accepted on March 20, 2003.