Year: 2008 Vol. 74 Ed. 5 - (4º)
Artigo Original
Pages: 657 to 661
Otorhinolaryngological and esophageal manifestations of epidermolysis bullosa
Author(s): Rodrigo Santana Fantauzzi1, Mariana Oliveira Maia2, Flávia Coelho Cunha3, Rodrigo Vidal Simões4, Denise Utsch Gonçalves5, Amélio Ferreira Maia6
Keywords: epidermolysis bullosa, esophagus, manifestations, otorhinolaryngology, treatment.
Abstract:
Epidermolysis bullosa (EB) is a group of skin diseases with different clinical manifestations and varied inheritance patterns. Blisters may appear spontaneously or following minimal trauma to the skin or mucosa. Aim: this paper aims to describe the otorhinolaryngological manifestations and esophageal complications related to EB, and the experience in treating patients with esophageal stenosis secondary to this disease. Materials and method: this descriptive study enrolled 60 patients with EB seen from June 1999 to December 2006 at the Head and Neck Surgery Service of X Hospital, a reference center for EB. Results: the patients' mean age was 14.5 years. Twenty-eight (46.6%) were females and 32 (53.4%) were males. Eight (13.4%) were diagnosed with epidermolysis bullosa simplex, while 51 (85%) had epidermolysis bullosa dystrophica; one (1.6%) patient had one acquired EB. Lips, mouth, tongue and ears were the most frequently involved sites (32 patients - 53.3%). Dysphagia was found in 28 patients (46.6%). After esophageal dilatation the symptoms subsided. Conclusion: EB is a rare disease and patients must be sent for treatment at reference centers. Physicians treating patients for EB must be aware of the measures required to improve the quality of the treatment provided without putting the patients in harm's way.
![]()
INTRODUCTION
Epidermolysis bullosa (EB) is a group of skin diseases with different clinical manifestations and varied inheritance patterns1-4. Blisters may appear spontaneously or following minimal trauma to the skin or mucosa4,5. EB affects one in every 50,000 livebirths4. Blisters appear secondary to the formation of cleavage planes in skin and mucosa2,3,5-7. EB can be divided into three large clinical groups: EB simplex (dominant autosomal) with blisters located in the epidermis that do not leave scars; junctional EB (recessive autosomal) in which blisters form in the lamina lucida; and EB dystrophica (dominant or recessive autosomal) characterized by the presence of atrophy, milium cysts, ungueal dystrophies, pigmentary alterations and mucosal injuries2,4.
Twenty-three phenotypes of EB are known, with varied clinical manifestations ranging from mild to lethal cases4. Diagnosis is done based on lesion clinical findings and pathology tests4. Electronic microscopy is the golden standard for diagnosis, but immunohistochemistry (monoclonal antibodies specifically) and molecular biology have provided sophisticated definitions of normal skin dermoepidermal junction structure and function and alterations found on each EB group and subgroup4.
Otorhinolaryngological manifestations are frequent in EB6-11. Such manifestations are related to the appearance of mucosal blisters (mainly in the oropharynx and esophagus), followed by rupture and hypertrophic scarring, leading to frenulum shortening, microstomia, esophageal stenosis, laryngeal stenosis, and nasal vestibule stenosis. Esophageal stenosis is a complication usually found in advanced cases, and remains as one of the main challenges in treating this condition12. Descriptive studies analyzing this infirmity have not yet been published in Brazil.
This paper aims to describe the otorhinolaryngological manifestations of epidermolysis bullosa, defining the best clinical and/or surgical approaches to consequently reduce patient morbidity and mortality.
MATERIALS AND METHOD
This is a descriptive study that enrolled 60 EB patients followed and treated from June 1999 to December 2006. The project was approved by the Ethics Committee of our institution under permit 136/05.
All patients were referred to the study by the Minas Gerais Association of Families, Friends, and Carriers of Epidermolysis Bullosa (AMPAPEB).
The 60 patients underwent a thorough otorhinolaryngological examination. Videofluoroscopy and contrast-enhanced x-rays of the esophagus, stomach, and duodenum were taken.
Esophageal dilatation was required in selected cases. Patients referred to the procedure had clinical and radiologic evidences of severe esophageal stenosis. Esophageal dilatation was performed using olives. All procedures were conducted with general anesthesia with propofol, succinylcholine, and midazolam. The procedures were carried out under apnea and mask ventilation until the patients recovered. After the procedure patients were prescribed steroids between 0.5 and 1mg/kg/5 days, analgesic drugs (paracetamol or dipyrone) and ranitidine (150mg/10ml) or omeprazole (20mg/12/12hs) for 30 days. Treatment course was prolonged depending on the presence of dyspeptic symptoms. Patients experiencing choking and dyspeptic symptoms without significant dysphagia took proton pump blockers or H2 receptor blockers in the previously prescribed dosages.
RESULTS
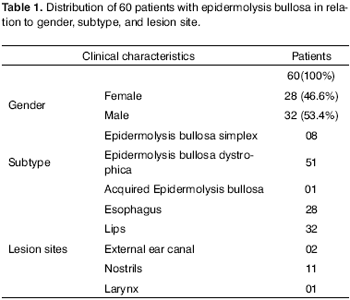
Twenty-eight (46.6%) of the 60 patients enrolled in the study were females, whereas 32 (53.4%) were males. Median age was 14.5 years and time of symptom onset varied between birth and 22 years of age.
Eight (13.4%) were clinically diagnosed with EB simplex, 51 (85%) with recessive EB dystrophica and one (1.6%) with acquired EB.
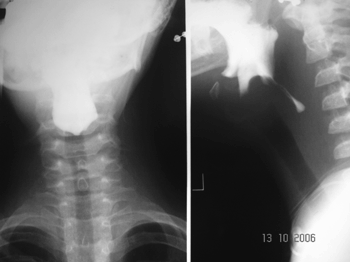
Esophageal manifestations were found in 28 (46.6%) patients. All patients underwent esophageal dilatation after esophageal stenosis was confirmed through x-ray images (Fig. 1).
Figure 1. Esophageal stenosis in the cricoid constriction
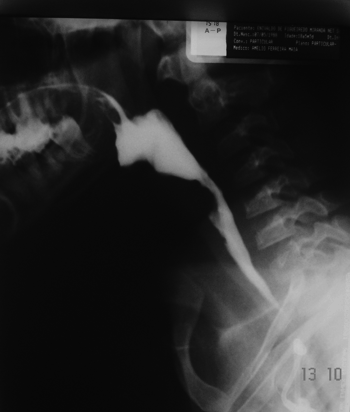
Two esophageal complications were observed: one patient submitted to dilatation evolved to an esophageal blister post-operatively and had to be hospitalized and clinically treated until the blister burst and the patient was able to swallow. Another patient had an esophageal perforation that evolved to pneumomediastinum and pneumoperitonium from the carina to the transverse mesocolon. This patient complained of intense retrosternal and chest pain while performing respiratory movements post-operatively. Chest x-rays were done to no diagnostic avail. A chest CT scan done subsequently showed a perforation. After starting treatment with clindamycin and gentamicin, the patient was referred to a drainage procedure covering the chest, mediastinum, and abdomen (retroperitonium). The patient evolved well and was discharged 30 days later. Dysphagia status was improved for all patients submitted to esophageal dilatation (Fig. 2).
Figure 2. Patient in the previous figure after dilatation with metal olives
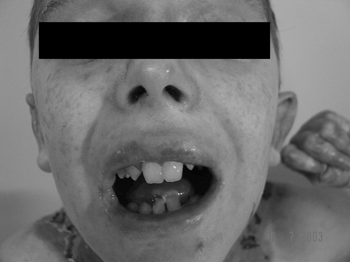
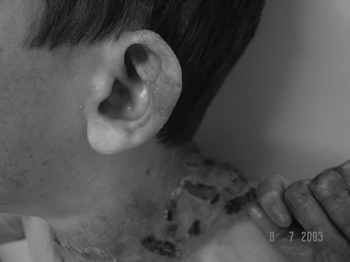
Lips, mouth, tongue, and ear were the most frequently involved sites (Fig. 3 and 4), with blisters found in 32 (53.3%) patients. Blisters were found in the external ear canal of two (3.3%) patients; none of them had signs of stenosis in the ear canal. Ulcerative lesions and nostril blisters were encountered in 11 (18.3%) patients, three (27.2%) of which evolved to nostril stenosis. One (1.6%) patient had laryngeal-tracheal alterations (anterior commissure stenosis) (Table 1).
Figure 3. Microstomia, shortened frenulum, perioral and oral mucosal ulcerations
Figure 4. Scar lesion in the ear (milium).
No complications related to anesthesia were observed, and none of the patients developed skin lesions after the procedures.
DISCUSSION
Epidermolysis bullosa is observed in a heterogeneous set of systemic diseases characterized by abnormal skin and mucosal frailty3. Research has recently shed light on important genetic defects4. Mutations on collagen type VII have been seen in cases of recessive and dominant EB dystrophica, while EB simplex has been associated with mutations on genes for keratin 5 or 143. Investigation has shown that some forms of junctional EB are caused by mutations in the laminin gene3. These findings have practical implications in the development of accurate pre-natal diagnosis and gene therapy3.
Depending on the type of epidermolysis bullosa, patients may develop phlyctenula in their mouths, esophagus, nostrils, ears, and larynx, resulting in significant morbidity6-11,13-15. Iron deficiency anemia is common with the blood losses subsequent to blister rupture7.
In relation to extracutaneous involvement, the upper gastrointestinal tract is the most frequently involved site7,10. Phlyctenula and microstomia may occur and lead to dysphagia and odynofagia7. Radiologic findings include stenosis, blisters, uneven mucosal surface, and hiatal hernia. The esophageal mucosa may be extremely fragile in patients with EB dystrophica, and perforation has been reported after endoscopy10.
Esophageal blisters usually evolve to scar tissue and stenosis and may secondarily lead to dysphagia, one of the biggest problems in patients with recessive EB dystrophica7. Complications such as esophageal perforation, pneumomediastinum, pneumoperitonium, and mediastinitis must be diagnosed as early as possible, as they may be fatal conditions12.
Teeth are often compromised in EB patients7,14,16. Preventive care is needed and frequent visits to a dental care provider are required to keep the teeth clean. Early fluorine application is indicated for pediatric patients7,14,16. All patients included in our study had poor dental status. EB patients have a hard time brushing their teeth, as even minimal trauma results in lesions.
Laryngeal involvement is rare. It occurs more frequently in junctional EB than on EB dystrophica or EB simplex8,9,13,17. Acute laryngeal involvement by blisters evolving to hoarseness and stridor requires immediate otorhinolaryngological intervention and possibly a tracheostomy2.
EB patients may be offered regular anesthesia, however with greater risk for complication17,18. A recent review looked at 129 anesthesia administrations in 32 EB patients and found no complications in patients using orotracheal intubation (10 cases), face mask, nerve blockage, intramuscular or intravenous agents, and local anesthesia. Similarly, no intubation-related complications were found in another report including 113 cases of oral intubation and 18 of nasal intubation done on 33 EB patients17. It is possible that the pseudostratified columnar epithelium that covers most of the larynx and trachea reduces the formation of friction blisters17. Despite their low risk of complication, any instrument that contacts skin and mucosa (face mask, laryngoscope, and endotracheal tubes) must be well lubricated17. Lubricated gauze has been indicated to fixate electrodes in cardiac monitoring, pressure cuff and venous accesses17.
Complications such as esophageal perforation, pneumomediastinum, pneumoperitonium, and mediastinitis must be diagnosed early, as they may be fatal12.
CONCLUSION
EB is a rare disease and patients must be sent to reference centers for treatment. Therefore, it is fundamental that the physicians involved in providing care to these patients are aware of the necessary measures to optimize the treatment without further harming the patients.
REFERENCES
1. Kowalewski C, Hamada T, Wozniak K, Kawano Y, Szczecinska W, Yasumoto S, et al. A novel autosomal partially dominant mutation designated G476D in the Keratin 5 gene causing Eidermolysis Bullosa simlex Weber-Cockayne type: A family study with a genetic twist. Int J Mol Med 2007;20(1):75-8.
2. Mitsuhashi Y, Hashimoto I. Genetic abnormalities and clinical classification of Epidermolysis Bullosa. Arch Dermatol Res 2003; 295 Suppl 1:529-33.
3. Fine JD, McGrath J. Inherited epidermolysis bullosa comes into the new millennium: A revised classification system based on current knowledge of pathogenetic mechanisms and the clinical, laboratory, and epidemiologic findings of large, well-defined patients cohorts. J Am Acad Dermatol 2000;43:135-7.
4. Bauer EA, Briggaman RA, Hintner H. Revised classification system for inherited epidermolysis bullosa. J Am Acad Dermatol 2000;42:1051-66.
5. Wojonarowska F, Eady RAJ, Burge SM. Bullous Eruptions. In: Champion RH, Burton JL, et al, editors. Rook/Wilkinson/ Ebling, Textbook of dermatology. 6th ed. Oxford: Blackwell Scientific; 1998. p. 1817-44.
6. Hore I, Bajaj Y, Denyer J, Martinez AE, Mellrio JE, Bibas T, et al. The management of general and disease specific ENT problems in children with Epidermolysis Bullosa - a retrospective case note review. Int J Pediatr Otorhinolaryngol 2007;71(3):385-91.
7. Johnston DE, Koehler R, Balfe DM. Clinical manifestations of epidermolysis bullosa dystrophica. Dig Dis Sci 1981;26(12):1144-49.
8. Liu RM, Papsin BC, de Jong Al. Epidermolysis Bullosa of the head and neck: A case report of laryngotracheal involvement and 10 - year review of cases at the Hospital for Sick Children. J Otolaryngol 1999; 28(2):76-82.
9. Schaffer S. Head and neck manifestations of epidermolysis bullosa. Clin Pediatr{Phila} 1992; 31: 81-8.
10. Ramadass T, Thangavelu T. Epidermolysis bullosa and its ENT manifestations. J Laryngol Otol 1978;92:441-6.
11. Thawley ES, Black JM, Dudek ES, et al. External auditory canal stricture secondary to epidermolysis bullosa. Arch Otolaryngol 1977;103:55-7.
12. Castillo RO, Davies YK, Lin YC, Garcia M, Young H. Management of esophageal strictures in children with recessive dystrophic Epidermolysis Bullosa. J Pediatr Gastroenterol Nutr 2002;34(5):535-41.
13. Kao CH, Chen SJ, Hwang B, Yang AH, Hsu CY, Huang CH. Junctional Epidermolysis Bullosa. J Chin Med Assoc 2006;69(10):503-6.
14. Silva LC, Cruz RA, Abou-Id LR, Brini LN, Moreira LS. Clinical evaluation of patients with Epidermolysis Bullosa: review of the literature and case reports. Spec Care Dentist 2004; 24(1):22-7.
15. Kastanioudakis I, Bassioukas K, Ziavra N. External ear involvement in epidermolysis bullosa. Otolaryngol Head Neck Surg 2000;122:618.
16. Serro MC, Silvestre DFJ, Bagán SJV, Peñarrocha DM, Alio SJJ. Hereditary Epidermolysis Bullosa. Dental management of three cases. Med Oral 2001;6(1):48-56.
17. Lin AN. Management of patients with epidermolysis bullosa. Dermatologic Clinics 1996;14(2):381-87.
18. Iohom G, Lyons B. Anaesthesia for children with Epidermolysis Bullosa: A review of 20 year's experience. Eur J Anesthesiol 2001;18(11):745-54.
1 Otorhinolaryngologist, Member of the Clinical Team at Hospital Municipal de Contagem.
2 MD, Resident at the ENT Service at Hospital das Clínicas da UFMG.
3 Otorhinolaryngologist, Member of the Clinical Team at Hospital Júlia Kubitschek.
4 Otorhinolaryngologist, Member of the Clinical Team at Hospital Militar do Estado de Minas Gerais.
5 PhD, Adjunct Professor of the Department of Ophthalmology, Otorhinolaryngology, and Speech and Hearing Therapy at the UFMG Medical School, Otorhinolaryngologist and Full Advisor at the Graduate Program on Infectology and Tropical Medicine at the UFMG Medical School.
6 Otorhinolaryngologist, Head and Neck Surgeon, Coordinator of the Otorhinolaryngology and Head and Neck Surgery Clinic at Hospital Felício Rocho. Hospital Felício Rocho.
Send correspondence to: Clínica de Otorrinolaringologia e Cirurgia de Cabeça e Pescoço, Hospital Felício Rocho - Avenida do Contorno 9530 3º andar Prado Belo Horizonte MG 30110-934.
Phone number: +55 31 3292-8128.
This paper was submitted to the RBORL-SGP (Publishing Manager System) on 12 June 2007. Code 4596.
The article was accepted on 25 August 2007.