Year: 2003 Vol. 69 Ed. 2 - (7º)
Artigo Original
Pages: 186 to 191
Concentration of interleukine 1B in periodontal disease
Author(s):
Gastão Valle Nicolau[1],
Abrão Rapoport[2],
Maria Aparecida Scnder Selski[3]
Keywords: periodontal disease, interleukine 1b.
Abstract:
Introduction: Determine the role of interleukine - 1b (IL-1b) as intercellular mediator in generation, amplification and maintenance of inflammatory and immune responses in periodontal disease. Study Design: Prospective non-randomized. Material and Method: Assessment of concentration of 1L-1b in group I (20 samples of healthy people) and in group II (20 samples of patients with periodontal disease), employing Easia Kit (Medgenix 1L-1b), with results statistically analyzed through Student t Test. Results: In control group, media and standard deviation of 1L-1b concentration were 71,28 ± 280,05 pg/ml and in periodontal disease 3618,49 ± 2864,75 pg/ml. Conclusion: The increase of Interleucine 1b in periodontal disease distinguish from healthy oral mucosa.
![]()
INTRODUCTION
The imbalance in the functional barrier of oral gingival mucosa triggers inflammatory and immune reactions that damage the structures that support the teeth1, 2. Thus, from the initial layer of glucoproteins, host-specific serum type bacteria (aerobe and anaerobe) have developed, with toxins determining the potential pH reduction, redox and proteolytic enzymes and the consequent onset of bacterial plaque3, 4, 5. Thus, the products that originate from such microorganisms and their reactions with the host synthesize the small proteins that receive the generic name of cytokines (interleukins, tumor necrosis factor, growth factor, interferon and others). For full efficacy of cytokines, it is indispensable to have a link with specific cell receptors of cell itself (autocrine action), adjacent cells (paracrine cells) or distant targets (endocrine action). As to expressions, they are regulated by the onset of specific signals triggered after each new mRNA transcription and the consequent synthesis of new proteins. These cytokines act not only as inflammatory mediators, but also as cell division and differentiation regulators2, 6, 7. Even if only potentially, all oral cells can produce cytokines in the initial phase of periodontium pathology bacterial invasion, and they arise from monocyte migration, macrophages and lymphocytes, even though the healthy gum has a predominance of polymorphonuclear cells. Thus, there is the onset of tumor necrosis factor (TNF-a) with low concentrations8,9, which reduce the competent cells to produce interleukins 1, 6 and 8.
Interleukin 1b (IL-1b) and TNFa have the potential to produce collagenase and bone resorption, fibroblasts, osteoblastic cells and consequent production of IL-6 and IL-11, which in turn, mediate the production of IL-1b and TNF-a6,10,11,12.
To sum up, IL-1b together with TNF-a are probably the triggering factors of the inflammatory reactions that conduct to bone resorption and destruction in periodontal disease. Dosage of IL-1b demonstrates concentrations of gingival fluid ranging from 16.5 to 844 ng/ml 13,14,15, enabling a comparative study of the healthy mucosa with the periodontal disease by means of quantification.
MATERIAL AND METHOD
We selected 40 patients (by order of arrival) in the Ambulatory of Periodontics, Department of Stomatology, Federal University of Parana, during the month of November 2001, divided into two groups: I (20 patients with healthy gums) and II (20 adult patients with periodontal disease with pockets that ranged from 5 to 11 mm deep). As to gender, there were 5 women in group I and 23 in group II, aged 29 to 37 years, and 37 men aged 21 to 58 years. None of the patients had been previously treated of periodontal disease and they were no smokers at that time.
As to method, we used:
1) Pre-cut and standardized strips of quantitative filter paper (Gramnex 3893 blue).
2) Phosphate buffer (PBS).
3) Bovine serum albumin at 0.5% (BSA).
4) Commercial kit for determination of interleukin 1b concentration in gingival fluid - Elisa IL-1b (Easia MedgenixTM Diagnostics S.A. - Zoning Industriel B-6220 Fleurus - Belgium). As to the composition of the kit:
a. one polyvinyl plate with microbowls for 96 tests;
b. 03 curve standards;
c. control 104 pg/ml ± 25 and 480 pg/ml ± 100;
d. a vial with antibody anti- IL-1b - HRP;
e. a vial of TMB substrate (tetramethylbenzidine);
f. a vial of lavage buffer;
g. a vial of block solution (SO4H2);
h. three vials of buffer substrate;
i. three vials of diluting solution;
j. high quality distilled water;
k. precision pipettes of 50 ml, 200ml, 1 ml, 10 ml;
l. Kline agitator (Depron) 700 rpm;
m. Hyperion Micro Reader 3.
For the selection of samples, we chose and isolated with cotton the most affected posterior sextant for gingival fluid collection. After determining the depth of the pockets by investigation made 7 days before the collection of the test sample, after 12 hour fast period, with no mechanical or chemical oral hygiene in the period, we made the collection in one day. We aspirated continuously the saliva, collected the fluid from the gingival crevice by soaking the pre-cut strips in the crevices during 60 seconds, with minor traumas, discarding samples with blood. The strips were maintained in 400 ml phosphate buffer solution and serum albumin at 0.5%, at 8ºC, up to the beginning of the assay conducted 24 hours after the collection. We analyzed the control samples and periodontal pathologies using the Easia kit (Medgenix IL-1b), which is an enzymatic immunoassay that combines monoclonal antibodies and different epitopes of IL-1b. These antibodies (MABs 1) adhered to the plaque microwells and some additions are made to them (MAB2), marking with peroxidase (HRP) and its macrogen (TMB). After 2 hour incubation, there was a "sandwich" reaction - MABs1- IL-1b - MAB2 - HRP. Next, we used the pipette to place 200ml in each hole of the immunoassay plaque, incubating it in dark at room temperature under the action of Kline agitator at ± 500 rpm for 15 minutes. We added 50 ml of block solution to the microwells in which there were cromogen reactions and measured the optical density of each sample in the Hyperion reader in a wavelength of 490nm (reference filter 630 or 650). To that end, we built a calibration curve among the optical density and concentration of IL-1b of the six standards in the kit. The standardized concentrations were respectively 0, 58, 170, 562, 1119, 2298 pg/ml, mean optical density of, respectively, 0.007, 0.0695, 0.215, 0.689, 1.296, 2.147 and the correlations were directly proportional (r = 0.9999116308; a = -1.7456206 and the regression coefficient b = 816.8842957). Thus, we determined the IL-1b concentrations by linear regression, such as correlation of optical density level of the diluting agent (3.973). We corrected the values calculated by degree of dilution of the respective samples, expressing the final values in pg/ml16.
As to the statistical analysis, we compared groups I and II by analysis of variance and tested the hypothesis that there were differences between the 2 groups by t Student test, with the conclusions resultant from p value.
RESULTS
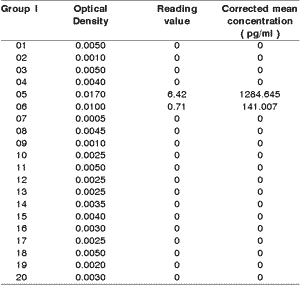
In the samples of gingival fluid of 40 people, we found undetermined levels of IL-1b in 18 of the 20 samples of group I. We considered undermined value whenever the optical density was equal or below 0.007 (mean value, whose extremes were: 0.005 and 0.009), because these values were measures in standards of interleukin, in which only the dilution fluid was present. Even though the gums of the group were uniformly healthy, two of the samples had dosage of IL-1b (Table 1).
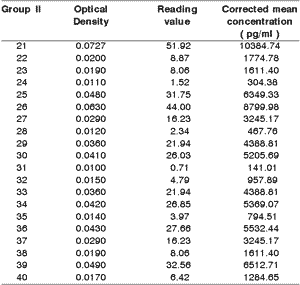
The corrected mean concentrations of IL-1b in the samples of group II were significantly greater (p<0.05) compared to the healthy group (Table 2). Despite the clinical uniformity comparing the degree of periodontitis, about 45% of the samples obtained in the respective gingival crevices had a relatively low value of IL-1b.
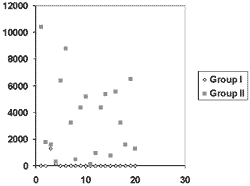
The distribution of values both in group I and in group II are demonstrated in Figure I.Table 1. 1b interleukin dosage in group I.
mean and standard deviation: 71.28 ± 280.05
Variance: 78428.58
Table 2. 1b interleukin dosage in group II.
Mean and standard deviation: 3618.49 ± 2864.75 pg/ml
Variance: 8206813.89
Difference between groups I and II: p < 0.05
Figure 1. Distribution of values of samples obtained in both groups.
DISCUSSION
In the present study, we gathered all details capable of increasing uniformity of gingival fluid collection. Despite all the care, however, in group II the dispersion of data was remarkable, from 0.141 to 10.385 ng/ml, which was translated into a standard deviation of 2.865 for 3.619 ng/ml of arithmetic mean. Similarly, in similar clinical circumstances, each author that uses commercial ELISA kits finds remarkable dispersion of data. Thus, we detected levels of IL-1b that had nullity at 97ng/ml16,17, as well as values of 313 ± 192 ng/ml18. However, low values were recorded19 with extremes of 0.04 and 0.240 ng/ml. We should understand this behavior in some groups in part as the precision index of determination of IL-1b of the samples. The capacity to determine IL-1b can be one of the most important technical causes for the fluctuation of the means of the various groups in the literature and for great data dispersion. We can understand and compare better what is exposed when we transfer the data of dispersion into percentage, as a coefficient of variation. Thus, the groups with the highest determination rates of IL-1b in samples had dispersion percentages that varied in the literature from 19% to 25% 13, 20, increasing progressively to 86%13, 15, 16, 17, 18, an inversion proportional to the recovery rate. There are exceptions, but the variation coefficient of 63% in found in populations with 100% recovery, probably because of the extremes of 131.8 and 843.818. The same applied to group II of the present study, whose variation coefficient was 79% for 100% recovery owing to the density of values close to the extremes. There are, therefore, 15% of the sample concentrations that are very low for IL-1b, resulting from optical density close to the range of 0.005 and 0.009, which in the calibration curve, corresponded to the sample with no IL-1b. The amplitude of fluctuation between these two values is about 45%, meaning that in the lower extreme of the curve the precision is excessively reduced. In the other levels of optical density, fluctuations in reading are between 0.43% and a maximum of 8.44%. It is possible that smaller dilutions than those recommended by the manufacturer of the kit would result in more precise reading, in lower concentrations that those used in studies16 within the extremes of the sensitivity curve, which ranges from 20 to 1000pg/ml.
The concern with the dispersion of IL-1b concentrations are connected with a greater level of difficulty than the comparison with individual clinical data, a situation that is well explained when we demonstrate the absence of correlation between IL-1b and depth of gingival crevice, when considering the studied population16. Therefore, it may be easy to demonstrate the tendency by means of IL-1b means as groups, but discrimination between health and disease in various degrees of periodontal impairment and the evolution assumption are not so easy to be demonstrated in isolated people, or in each one of the sites of the same arch21. In group II of our study, in which dispersion of IL-1b concentration reached an average of 79%, each value of interleukin originated from gingival crevice fluid that, as a whole and as a group, represented an uniform sampling, restricted by its depth, equal or greater than 5mm, accepted as a synonym of stable and advanced periodontitis 22, whose estimated bone loss was between 30 and 69%19, 20. Despite the binary behavior of the present sample, shared by others20, in which undetermined IL-1b occurs in healthy gums and elevated levels are detected in the group with periodontitis, there are about 30% of the data that are overlapping. It means that in 70% of the cases, the distinction between healthy gums and periodontitis is easy in individual cases, but in 30% of the times, the doubt still remains. It is even more difficult to stratify each case in levels of periodontitis or assess the corresponding bone loss only through the dosage of IL-1b, as observed previously23. It is possible that for such reasons, many authors found a strong and direct correlation of the various levels of periodontitis only with isolated clinical data, such as depth of crevice, volume of gingival fluid, loss of periodontal adherence, gingival index, plaque index23, 24. Some researchers19, 20, 25, however, correlated direct and significantly IL-1b concentration or its corresponding genotypes with clinical parameters and the level of bone destruction19, 20, 25 so as to make a table with six levels of IL-1b, between 50 and 230 pg/ml and degrees corresponding to bone loss, between 0.5 and 3mm. Other researchers16, 22, 26, 27 pointed towards an increase in IL-1b as a circumstantial aspect for coinciding with depth of the gingival crevice, but it does not correspond to degree of periodontal impairment. What reinforces this concept is the confirmation that when it is periodontitis, there is an attenuation of the clinical parameters, whereas the levels of interleukin are unaltered for six months. The reduction of IL-1b levels after this period translates into the probable persistence of the residual inflammatory process, part of the process of recovery or the association of the dental plaque as still present24. Conversely, in human experimental gingivitis, there is an initial peak of IL-1b concentration that continues in a level that is somewhat constant, whereas the intensity of gingival inflammation and deepening of the crevice still progress17. For this reason, we can state26 that the level of IL-1b in the gingival fluid assesses the number of gingival cells that produce it, since the correlation is strong and direct. In group I, in which 18 samples had undetermined IL-1b, we found a sample with low value and another one with surprisingly high value compared to the other ones. There is no way to precise the causes of such fact in the study, but in both cases, the consequences indicate a strong family tendency to the affection: parents and siblings had severe periodontal disease, with loss of teeth. Other causes, though, are not excluded and include residual behavior of interleukin in a resolution process.
We classified the representatives of group II as having periodontitis with exsudate, in whose gingival crevices there was a continuous migration of neutrophils owing to the presence of microorganism products (fMet-Leu-Phe, LPS and others) and the presence of IL-8, complement C5a, LTB4, PGE2, TxB2 and auxiliary adhesion molecules such as ICAM-1 and ELAM-1 and other adhesines. Those molecules contributed for PMN to infiltrate the coronal, junctional and apical aspects of the tissues, and the other immune-inflammatory cells remain in the tissue28. In such circumstances, the plaques are larger in the apical direction, in which they develop an environment anaerobe niche. Some macrophages migrate to the region. This infiltrate expands laterally and towards the connective tissue, in which there is increase density of macrophages, lymphocytes and a large number of plasmocytes. In this phase, plasmocytes reach 51% of the areas of greatest activity, whereas in regions of least activity they reach only 30% of the section. Therefore, greater infiltrate of lymphocytes B corresponds to more or less stable disease, when the number of T cells predominates. At the same time the cell infiltrate increases in the connective tissue, especially in the subepithelial area, it reduces the number of fibroblasts and the quantity of collagen28, 29, 30.
CONCLUSIONS
It is concluded that despite the fact that concentrations of IL-1b can easy separate groups I and II. and represent a second population group that is relatively uniform, many values of cytokines overlap the data of group I. The use of the concentration of one cytokine to define the degree of periodontitis creates a relation that is only circumstantial with the clinical data, being a fragile contribution to the diagnosis. It is possible that the concomitant dosage of other factors such as products that indicate the number of cells, for example, myeloperoxidase, b-glucuronidase, or its activity, such as metalloproteinases, ILs-1, IL-1ra, IL-6, IL-8, prostaglandins, TGFb or even cells such as T CD3+ and the proportion of the subgroups CD4+ and CD8+ can contribute to detailing and understanding the various phases of periodontitis. For this reason, the studies should continue with more data so that we can define or even create new rates that can make it more precise for the classification and prognosis of various levels of periodontitis. In summary, the dosage of interleukin 1b in gingival crevice fluid enabled the distinction between the group with normal and affected periodontium, respectively groups I and II.
REFERENCES
1. Williams DM. Gengivitis and periodontal disease. In: McGee JO', Isaacson PG, Wright NA. (Ed.) Oxford textbook of pathology. Oxford: Oxford University Press; 1992. v.2a., p.1091-96.
2. Abbas AK, Lichtman AH, Pober JS. Cellular and molecular immunology. 2nd. Philadelphia: Saunders; 1994. 457p.
3. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms. A common cause of persistent infections. Science 1999;284:1318-22.
4. Kadono H, Kdo J-I, Kataoka M, Yamaguchi N, Nagata T. Inhibition of osteoblastic cell differentiation by lipopysaccharide extract from Porphyromonas gigibalis. Infect Immun 1999;67:2841-46.
5. Costerton JW, Stewart PS. Battling biofilms. Scient American 2001;285:61-7.
6. Howells GL. Cytokine networks in destructive periodontal disease. Oral Dis 1995;1:266-70.
7. Hyde RM. Immunology. 3rd. Philadelphia: Williams & Wilkins; 1995. p.316.
8. Matsuki Y, Yamamoto T, Hara K. Localization of interleukin-1 (IL-1) mRNA-expressing macrophages in human inflamed gingiva and IL-1 activity in gingival crevicular fluid. J Periodont Res 1993;28:35-42.
9. Agarwal S, Baran C, Piesco NP, Quintero JC, Langkamp HH, Johns lP, Chandra CS. Synthesis of proinflammatory cytokines by human gingival fibroblasts in response to lipopolysaccharides and interleukin-1b. J Periodont Res 1995;30:382-9.
10. Hillmann G, Hillmann B, Geursten W. Immunohistological determination of interleukin-1 beta in inflamed gingival epithelium. Arch Oral Biol 1995;40:353-9.
11. Yucel-Lindberg T, Lerner UH, Modéer T. Effects and interactions of tumour necrosis factor a and bradykinin on interleukin-1 production in gingival fibroblasts. J Periodont Res 1995;30:186-91.
12. Manolagas SC. Role of cytokines in bone resorption. Bone 1995;17(2):635-75.
13. Payne JB, Reinhardt RA, Masada MP, Dubois LM, Allison AC. Gingival crevicular fluid IL-8: correlation with local IL-1b levels and patient estrogen status. J Periodont Res 1993;28: 451-3.
14. Johnson GK, Poore TK, Squier CA, Wertz PW, Reinhardt RA, Vincent SD. Prostaglandin E2 and interleukin-1 levels in smokeless tobacco-induced oral mucosal lesions. J Periodont Res 1994;29:430-8.
15. Lamster IB, Pullman JR, Celenti RS, Grbic JT. The effect of tetracycline fiber therapy on b-glucoronidase and interleukin1-b in crevicular fluid. J Clin Periodontol 1996;23:816-22.
16. Wilton JMA, Bampton JLM, Griffiths GS, Curtis MA, Life JS, Johnson NW, Powell JR, Harrap GJ, Critchley P. Interleukin-1 beta (IL-1b) levels in gingival crevicular fluid from adults with previous evidence of destructive periodontitis. J Clin Periodontol 1992;19: 53-7.
17. Heasman PA, Collins JG, Offenbacher S. Changes in crevicular fluid leves of interleukin-1b, leukotriene B4, prostaglandin E2, thromboxane B2 and tumour necrosis factor a in experimental gingivitis in humans. J Periodont Res 1993;28:241-7.
18. Preiss DS, Meyle J. Interleukin-1b concentration of gingival crevicular fluid. J Periodontol 1994;65:423-8.
19. Cavanaugh PF, Meredith MP, Buchanan W, Doyle MJ, Reddy MS, Jeffcoat MK. Coordinate production of PGE2 and IL-1b in the gengival crevicular fluid of adults periodontitis. J Periodont Res 1998;33:75-82.
20. Ishihara Y, Nishihara T, Kuroyanagi T, Shirozu N, Yamagishi E, Ohguchi M, Koide M, Ueda N, Amano K, Noguchi T. Gingival crevicular interleukin-1 and interleukin-1 receptor antagonist levels in periodontically healthy and diseased sites. J Periodont Res 1997;32: 524-9.
21. Williams RC, Paquete DW. Avanços no diagnóstico periodontal. In: Lindhe J, Karring T, Lang NP. (Ed.) Tratado de periodontia clínica e implantologia oral. 3.ed. Rio de Janeiro: Guanabara-Koogan; 1999. p. 281-98.
22. Reinhardt RA, Masada MP, Kaldhal WB, Dubois LM, Kornman KS, Choi JI, Kalkwarf KL, Allison AC. Gingival fluid IL-1 and IL-6 levels in refractory periodontitis. J Clin Periodontol 1993;20:225-31.
23. Wollf LF, Koller NJ, Smith QT, Mathur A, Aeppli D. Subjingival temperature. J Clin Periodontol 1997;24: 900-6.
24. Alexander DCC, Martin JC, King PJ, Powell JR, Caves J, Cohen ME. Interleukin-1 beta, prostaglandin E2, and immunoglobulin G subclasses in gingival crevicular fluid in patients undergoing periodontal therapy. J Periodontol 1996;67: 755-62.
25. McDevitt MJ, Wang H-Y, Knobelman C, Newman MG, Di Giovine FS, Timms J, Duff GW, Kornman KS. Interleukin-1 genetic association with periodontitis in clinical practice. J Periodontol 2000;71:156-63.
26. Feldner BD, Reinhardt RA, Garbin CP, Seymour GL, Casey JH. Histological evaluation of interleukin-1b and collagen in gingival tissue from untreated adult periodontitis. J Periodont Res 1994;29:54-61.
27. Shimauchi H, Takayama S, Imai-Tanaka T, Okada H. Balance of interleukin-1b and interleukin recptor agonist in human periapical lesions. J Endodont 1998;24:116-9.
28. Roberts FA, Hockett Jr RD, Bucy RP, Michalek SM. Quantitative assessment of inflammatory cytokine gene expression in chronic adult periodontitis. Oral Microbiol Immunol 1997;12:336-44.
29. Yamazaki K, Nakajima T, Kubota Y, Gemmell E, Seymour GJ, Hara K. Cytokine messenger RNA expression in chronic inflammatory periodontal disease. Oral Microbiol Immunol 1997;12:281-7.
30. Kinane DF, Lindhe J. Patogênese da periodontite. In: Lindhe J, Karring T, Lang NP. (Ed). Tratado de periodontia clínica e implantologia oral. 3ª ed. Rio de Janeiro: Guanabara:Koogan; 1999. p.127-52.
1 Master in Health Sciences, Hospital Heliópolis, Hosphel, São Paulo, SP.
2 Full Professor, Head and Neck Surgery, Hospital Heliópolis, Hosphel, SP.
3 Professor of the Specialization Course in Periodontics, UFPR.
Study conducted at the Department of Stomatology, Federal University of Parana, Department of Head and
Neck Surgery and Otorhinolaryngology, Hospital Heliópolis, Hosphel, São Paulo.
Address correspondence to: Prof. Dr. Abrão Rapoport - Rua Iramaia, 136 Jd. Europa 01450-020 - São Paulo SP
Tel (55 11) 273-8224 - E-mail: cpgcp.hosphel@attglobal.net
Article submitted on December 09, 2002. Article accepted on February 13, 2003.